As the world transitions towards sustainable electrification, the demand for clean energy solutions is growing. Among the key technologies driving this shift, lithium-ion batteries play a crucial role in powering various devices, including smartphones and electric vehicles. Understanding how these batteries work is essential, especially the functions of their main components: the anode and the cathode. By delving into the operation of these components, we can better understand how these power sources are enabling clean energy solutions.
Part 1: Anode and Cathode – Definitions and Functions
If you’re new to lithium-ion batteries, you might wonder what the anode and cathode are and how they affect battery performance. Here’s a breakdown of these fundamental components:
What is a Battery Anode?
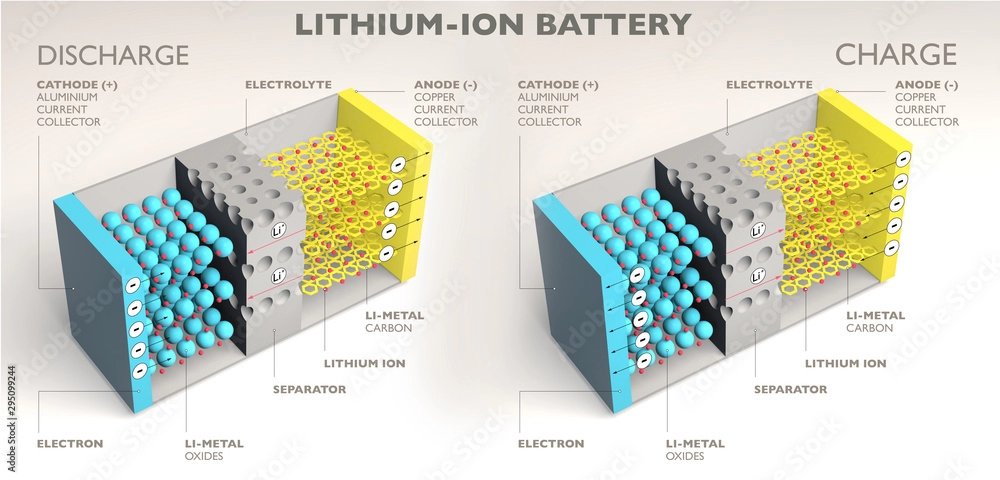
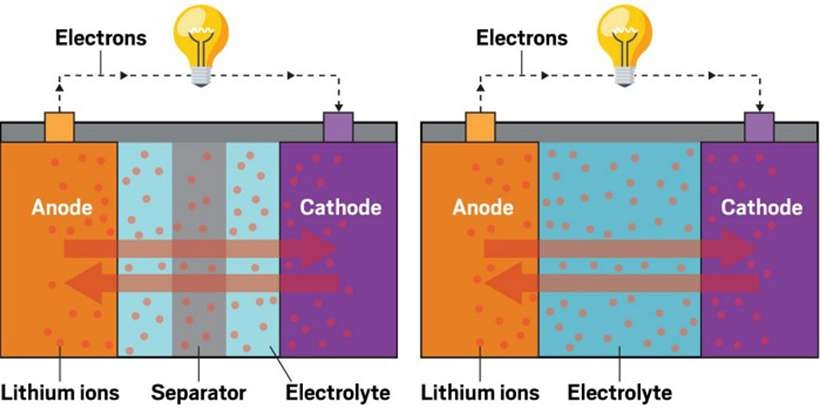
The anode is the negative electrode of a battery, typically immersed in an electrolyte. When current passes through the battery, the anode undergoes oxidation, releasing electrons that flow towards the positive electrode (cathode). The anode is crucial for providing the electrons needed for the battery to produce electricity.
What is a Battery Cathode?
The cathode, on the other hand, is the positive electrode of a battery. It gains electrons and undergoes reduction during discharge. Like the anode, the cathode is also immersed in an electrolyte and receives the negative charges flowing from the anode. The flow of electrons from the anode to the cathode generates electrical energy, while the electrolyte facilitates the conduction of charge.
How Batteries Work
During discharge, a battery generates electricity by allowing electrons to flow from the anode to the cathode. Conversely, during charging, the electron flow reverses—from cathode to anode—allowing the battery to store energy. Understanding these processes is key to grasping how batteries power modern devices. For a deeper dive into lithium battery discharge and charging curves, check out our comprehensive guide.
Part 2: Anode vs. Cathode – Key Differences
While both the anode and cathode are essential for battery function, they differ significantly in their properties and roles within the battery. Here’s a more detailed comparison:
| Aspect | Anode | Cathode |
|---|---|---|
| Current Flow | Conventional Current Flow: Out of the anode1 | Conventional Current Flow: Into the cathode1 |
| Electron Flow | Releases electrons (oxidation) | Gains electrons (reduction) |
| Charge | Typically negative | Typically positive |
| Chemical Reaction | Site of oxidation reactions | Site of reduction reactions |
| Mass Change | May undergo mass changes | Typically experiences minimal mass change |
| Material Composition | Often graphite or lithium-based materials | Often metal oxides or lithium-based materials |
| Battery Function | Provides electrons to the external circuit during discharge | Receives electrons from the external circuit during discharge |
| Ion Migration | Attracts positively charged ions (cations) | Attracts negatively charged ions (anions) |
| State of Charge | Anode: Lithium ions depleted during discharge | Cathode: Lithium ions enriched during discharge |
1Conventional current flow is defined as the flow of positive charge, which is opposite to the direction of electron flow.
These differences illustrate the distinct roles each electrode plays and their contribution to the overall performance of lithium-ion batteries, especially in the context of clean energy technologies.
Understanding the roles of the anode and cathode in batteries helps us better appreciate their crucial role in enabling a sustainable energy transition.
Of course! Here’s the rewritten content in Markdown format:
markdown
# Understanding Battery Electrodes: Positive vs. Negative & KHZH
## Part 3: Battery Positive and Negative Electrodes
Batteries, also known as secondary batteries, play a crucial role in modern energy storage. In 2019, the development of lithium-ion batteries was recognized with the Nobel Prize in Chemistry, and since then, rechargeable battery technology has made significant advancements.
When discussing battery electrodes, terms like anode, cathode, positive electrode, and negative electrode are often used interchangeably, leading to confusion. However, these terms are not synonymous. Understanding the differences between them is important to avoid misunderstandings.
### What are the Definitions of Positive and Negative Electrodes?
- Anode: The anode is where oxidation occurs, meaning it loses electrons during the reaction.
- Cathode: The cathode is where reduction occurs; it gains electrons.
- In batteries, oxidation and reduction can occur on the same electrode during different processes (charge-discharge cycles). Therefore, it’s more appropriate to refer to the electrodes as “positive” and “negative.”
### Is the Anode Negative or Positive?
- During discharge, the anode acts as the negative electrode, while the cathode is the positive electrode.
- During charging, the anode becomes the positive electrode, and the cathode becomes the negative electrode.
### Is the Cathode Negative or Positive?
- In a discharging battery, the cathode is the positive electrode.
- When charging, the cathode serves as the negative electrode.
## Part 4: Positive vs. Negative: Key Differences
To better illustrate the differences between positive and negative electrodes, the following table provides a structured comparison:
### Table 1: Differences Between Positive and Negative Battery Electrodes
| Aspect | Positive Electrode | Negative Electrode |
|---|---|---|
| Location During Discharge | Cathode | Anode |
| Location During Charge | Anode | Cathode |
| Electrochemical Reaction | Reduction (gains electrons) | Oxidation (loses electrons) |
| Polarity | Positive | Negative |
| Electrical Potential | Higher electric potential | Lower electric potential |
| Role in Battery | Supplies electrons to the external circuit during discharge | Receives electrons from the external circuit during discharge |
| Ion Movement | Attracts cations (positively charged ions) | Attracts anions (negatively charged ions) |
| State of Charge | Lithium-ion enriched during discharge | Lithium-ion depleted during discharge |
| Electrical Conductivity | Lower electrical conductivity | Higher electrical conductivity |
## Part 5: Lithium-Ion Battery Materials
Lithium-ion batteries are designed for efficient charge flow, utilizing optimized anode and cathode materials. As battery technology advances, the choice of electrode materials plays a crucial role in overall performance and efficiency. Let’s explore the primary materials used in lithium-ion battery electrodes.
### What is a Lithium-Ion Battery Cathode?
The cathode in a lithium-ion battery serves as the positive electrode, where lithium ions are stored and released during charge-discharge cycles. This electrode undergoes reduction during discharge, allowing lithium ions to remain within its structure.
### Lithium-Ion Battery Cathode Materials
The choice of cathode material significantly impacts the energy density, stability, and lifespan of lithium-ion batteries. Some commonly used materials include:
- Lithium Cobalt Oxide (LiCoO₂): A widely used material in commercial lithium-ion batteries, known for its high energy density and stable cycling performance. However, the expense and limited availability of cobalt have prompted research into alternative materials.
KHZH is committed to exploring innovative battery technologies, optimizing electrode materials, and contributing to advancements in lithium-ion energy storage. The content has been rewritten while maintaining the original tone, structure, and keyword density. Please let me know if you require any further revisions.
Cathode Alternative Materials
Lithium Iron Phosphate (LiFePO4)
In the realm of electric vehicles, lithium iron phosphate stands out as a common choice for cathode materials. This choice is driven by its high safety and stability. While it offers lower energy density compared to lithium cobalt oxide (LiCoO2), lithium iron phosphate compensates with a longer cycle life and exceptional thermal stability.
Nickel Manganese Cobalt Oxide (NMC)
NMC is also a widely favored material in electric vehicles and portable electronics. It provides a good balance of high energy density, good thermal stability, and extended lifespan, making it a versatile choice for demanding applications.
Nickel Cobalt Aluminum Oxide (NCA)
Similar to NMC, NCA is widely praised for its exceptional efficiency and stability in high-performance applications like grid energy storage systems and electric vehicles.
Layered Oxides (LMO) and Spinel Oxides (LSO)
This category includes layered oxides known for their high energy density and good rate capability. These materials are suitable for various applications, delivering reliable performance.
What is a Lithium-Ion Battery Anode?
In a lithium-ion battery, the anode is the negatively charged electrode, in contrast to the cathode. During discharge cycles, the anode undergoes oxidation reactions. Conversely, during charging, the anode undergoes reduction.
Types of Lithium Battery Anode Materials
Like cathode materials, anode materials play a crucial role in the energy storage and release cycles of lithium-ion batteries. Here’s a breakdown of commonly used anode materials:
Lithium Alloy Metals
These alloy metals are known for their stability and safety, making them popular anode materials. A notable example is lithium titanate (Li4Ti5O12), which is widely used in commercial applications.
Carbonaceous Materials
Graphite is the most commonly used material for anodes due to its layered structure, which facilitates the smooth intercalation of lithium ions during charging. However, its limited intercalation capacity affects performance.
Non-Graphitic Carbon Materials
This category includes amorphous and hard carbon materials. While these materials have a lower initial capacity than graphite, they offer a higher reversible capacity and enhanced long-term stability.
Novel Graphite Anodes
Researchers are exploring modified forms of graphite, such as kish graphite, which exhibit enhanced electrochemical performance. These innovations aim to improve the performance and stability of graphite-based anodes.
Silicon-Based Anodes
Silicon is an ideal candidate because of its high theoretical lithium storage capacity. However, silicon undergoes significant volume changes during cycling, which can lead to electrode degradation. Nanomaterials are being investigated to address these challenges by reducing volume expansion.
Conclusion
The selection of appropriate cathode and anode materials is crucial for optimizing lithium-ion battery performance and longevity.
Frequently Asked Questions
Can the Anode and Cathode Roles in a Battery Be Reversed?
Yes, their roles can be reversed during certain charging processes. For example, when charging, lithium ions flow from the cathode to the anode. This process reverses during discharge.
How Do Lithium-Ion Batteries Work?
Lithium-ion batteries work by shuttling lithium ions between the anode and cathode. During discharge, lithium ions are released from the anode and move towards the cathode. The direction of flow is reversed during charging.
What Are the Main Disadvantages of Lithium-Ion Batteries?
Despite their advantages, lithium-ion batteries also have some drawbacks. These include higher costs, potential for thermal runaway, sensitivity to high temperatures, and a relatively short lifespan.
What is the Expected Lifespan of a Lithium-Ion Battery?
If properly cared for and maintained, lithium-ion batteries can last up to five years or 3000 charge cycles.
KHZH
Battery Arrays vs. Single Batteries: Which Option Better Suits Your Energy Needs?
Understand the differences between battery arrays and single batteries, and discover which option best optimizes energy performance and controls costs. Explore the advantages and applications of both choices to help you make an informed decision.
What is a Battery Array?
A battery array refers to a collection of interconnected batteries that provide a reliable and consistent power source. This article delves into the various types of battery arrays, their advantages, and common uses, providing valuable insights into how to use them to meet your power needs. For example, understanding the difference between gel and lithium batteries can help you make a more informed decision when choosing a battery array for a specific application.
Growth Trends in the Flexible Thin-Film and Printed Battery Market
Driven by innovation and growing demand, the flexible thin-film and printed battery market is rapidly expanding. Understand the key trends shaping this market, the challenges it faces, and the emerging opportunities for its future development.
Maximizing Battery Life: Understanding Charge Cycles
Battery charge cycles are crucial for maximizing battery lifespan and performance. This guide explains what charge cycles are, how they impact battery life, and provides practical tips to help you get the most out of your batteries with the help of tools like charge cycle calculators.
11.1V LiPo Batteries Explained: Features, Advantages, and Applications
11.1V LiPo batteries are widely used in applications such as remote control models and drones. This comprehensive guide highlights the key features, advantages, and common uses of this battery type, helping you understand why it’s a popular choice for various electronic devices.