Lithium-containing batteries, such as rechargeable batteries in cell phones, laptops, and electric vehicles, have now become an indispensable part of our daily lives. At the same time, research into lithium-based batteries has become a key area of scientific innovation. As a result, terms such as “lithium battery,” “lithium-ion battery,” and “lithium-metal battery” are frequently heard. But are they all the same thing?
Both lithium-metal batteries and lithium-ion batteries are types of lithium batteries. In this article, we will explain the differences between the two.
Part 1: Understanding Lithium-Ion Batteries
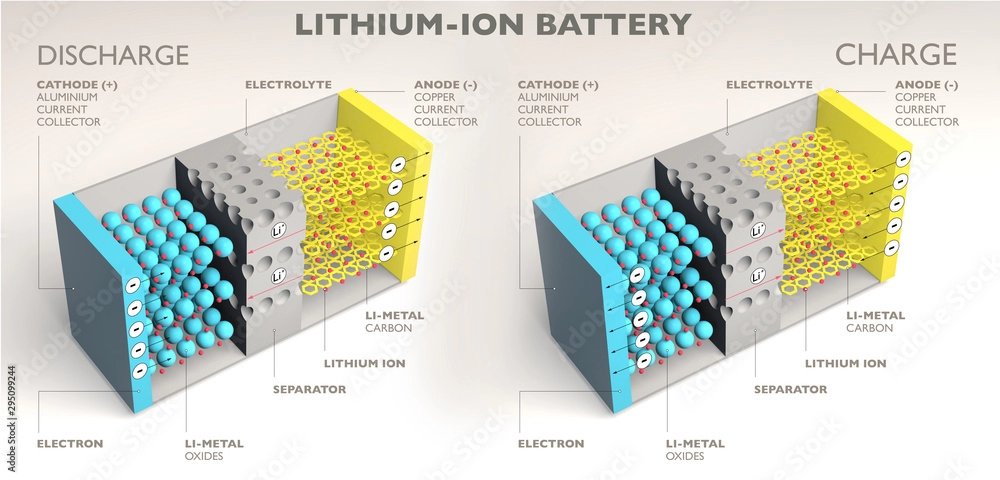
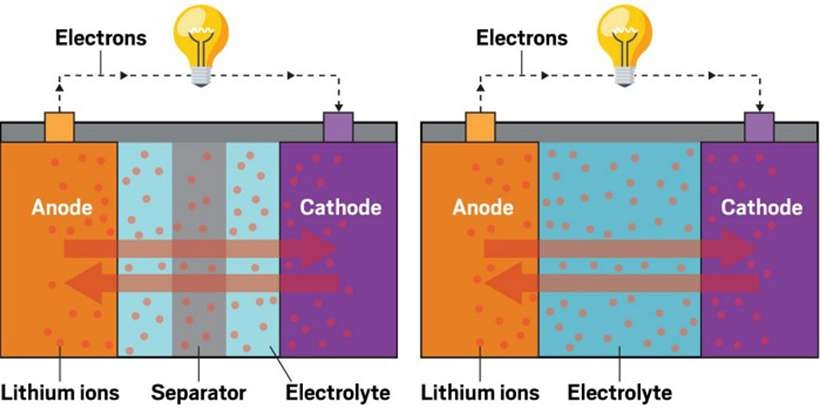
A lithium-ion battery is a type of secondary (rechargeable) battery that primarily works by moving lithium ions between the positive and the negative electrodes. During charge and discharge cycles, lithium ions (Li+) move back and forth between the electrodes. When a lithium-ion battery is charged, Li+ ions are extracted from the positive electrode and embedded in the negative electrode through the electrolyte. As a result, the negative electrode becomes enriched with lithium ions. During discharge, the opposite occurs.
1. Components of a Lithium-Ion Battery
A. Positive Electrode (Cathode)
The active material in the positive electrode is typically made of lithium manganese oxide, lithium cobalt oxide, or lithium nickel manganese cobalt oxide. For electric bicycles, lithium nickel manganese cobalt oxide (commonly referred to as ternary materials) or a combination of ternary with a small amount of lithium manganese oxide is often used. Pure lithium manganese oxide and lithium iron phosphate are becoming less popular due to their size, performance, or cost. The current collector is usually made of electrolytic aluminum foil with a thickness of 10-20 micrometers.
B. Separator
The separator is a specially manufactured polymer film with a microporous structure that allows lithium ions to pass freely but blocks the passage of electrons.
C. Negative Electrode (Anode)
The negative electrode uses graphite or graphite-based materials as the active material. The current collector is usually electrolytic copper foil with a thickness of a 7-15 micrometers.
D. Organic Electrolyte
The electrolyte is usually a carbonate-based solvent in which lithium hexafluorophosphate is dissolved. Lithium polymer batteries use gel electrolytes.
E. Battery Case
Lithium-ion batteries can have various case types, including steel cases (less common in square form), aluminum cases, nickel-plated iron cases (for cylindrical batteries), and aluminum-plastic film (in soft-pack batteries). The battery cover also functions as the positive and negative terminals.
2. How Lithium-Ion Batteries Work
Lithium-ion batteries use carbon materials as the negative electrode and lithium-containing compounds as the positive electrode. Unlike lithium-metal batteries, lithium-ion batteries do not contain lithium metal; instead, they contain lithium ions. This is the defining characteristic of a lithium-ion battery.
The working principle of lithium-ion batteries is based on the insertion and extraction of lithium ions. During charge and discharge cycles, lithium ions are inserted into or removed from the electrodes. This process is often referred to as a “rocking chair battery,” which vividly describes the reciprocating movement of lithium ions between the electrodes.
When the battery is charging, lithium ions are generated at the positive electrode. These lithium ions migrate through the electrolyte to the negative electrode. The carbon used in the negative electrode has a layered structure with many micropores, into which the lithium ions are embedded. The more lithium ions embedded in the carbon, the higher the battery’s charging capacity.
For more in-depth information on the performance and lifespan of lithium-ion batteries, click here.
Part 1: Understanding Lithium-Ion Batteries
Lithium-ion batteries are widely used in various devices, including mobile phones, laptops, and electric vehicles, due to their efficiency and longevity. These batteries consist of two electrodes—a negative electrode (anode) and a positive electrode (cathode)—and an electrolyte. During charging, lithium ions move from the cathode to the anode and are stored in carbon layers. During discharge, lithium ions move back to the cathode, releasing electrical energy.
The charging current for lithium-ion batteries is typically between 0.2C and 1C. A higher current allows for a faster charging process but also generates more heat. Charging with excessive current can prevent the battery from reaching its full capacity, much like pouring a beer too quickly results in excessive foam and wasted beer.
Advantages of Lithium-Ion Batteries
- High Voltage
A single lithium-ion battery can operate at voltages as high as 3.7-3.8V (3.2V for lithium iron phosphate batteries), which is about three times the voltage of nickel-cadmium and nickel-metal hydride batteries. - Specific Capacity
Lithium-ion batteries have an impressive specific capacity, with practical *specific* energy densities reaching around 555Wh/kg. The materials used in these batteries can achieve capacities of over 150mAh/g, which is 3-4 times that of nickel-cadmium batteries and 2-3 times that of nickel-metal hydride batteries, approaching 88% of their theoretical value. - Long Cycle Life
Lithium-ion batteries can typically last for over 500 charge cycles, and some can exceed 1,000 cycles. Lithium iron phosphate batteries, in particular, can last for over 2,000 cycles. This long cycle life enhances the market competitiveness of devices. - Safety
Lithium-ion batteries are safer than their predecessors, such as lithium metal batteries, which are prone to short circuits due to dendrite formation *of* lithium metal. Additionally, lithium-ion batteries do not contain environmentally harmful substances like cadmium, lead, and mercury, and they do not suffer from the “memory effect” like nickel-cadmium batteries. - Low Self-Discharge
A fully charged lithium-ion battery has a self-discharge rate of about 2% per month when stored at room temperature, which is much lower than the 25%-30% of nickel-cadmium batteries and the 30%-35% of nickel-metal hydride batteries. - Fast Charging
Lithium-ion batteries can be charged quickly, reaching over 80% of their nominal capacity after charging for 30 minutes at a 1C charge rate. Lithium iron phosphate batteries, in particular, can be charged to 90% of their capacity in 10 minutes. - Wide Operating Temperature Range
Lithium-ion batteries can operate in temperatures ranging from -25°C to 45°C. With advancements in electrolytes and cathode materials, this range may expand to -40°C to 70°C.
Disadvantages of Lithium-Ion Batteries
- Aging
Lithium-ion batteries slowly lose capacity over time, which is influenced by usage and temperature. This decline manifests as a decrease in capacity or an increase in internal resistance. - Failure Rate
Approximately 1% of new lithium-ion batteries need to be recycled due to various factors. - Susceptibility to Overcharging
Overcharging can cause excessive lithium ions to be embedded in the anode material, permanently damaging the battery’s internal structure and significantly shortening its lifespan. - Susceptibility to Over-Discharging
Over-discharging can lead to excessive extraction of lithium ions from the cathode material, potentially damaging the crystal lattice and reducing the battery’s lifespan.
Part 2: Lithium Metal Batteries
Lithium metal batteries (LMBs) are a type of battery that uses metallic lithium as the anode. The cathode can be composed of materials such as oxygen, sulfur, or metal oxides. Lithium metal batteries operate on principles similar to those of traditional batteries. The oxidation reaction of metallic lithium generates electrical energy, making LMBs a potential alternative for applications requiring higher energy density and performance.

Lithium Metal Batteries
Lithium metal batteries are non-rechargeable and cannot be recharged once depleted.
These batteries use metallic lithium as an electrode, allowing them to store more electrical energy compared to traditional dry-cell batteries. This high energy density makes them ideal for powering devices that require long-lasting energy, such as portable electronics like cameras.
What are Lithium Metal Batteries?
A lithium metal battery is a type of non-rechargeable (primary) battery that uses pure metallic lithium as an anode. Known for their high energy density and long shelf life, these batteries are well-suited for applications requiring a durable, compact, and long-lasting power source.
Key Features of Lithium Metal Batteries:
- High Energy Density:
Lithium metal batteries can store more energy compared to other types of batteries, allowing them to provide long-lasting power in a lighter, smaller package. - Non-Rechargeable:
These are primary batteries, meaning they are designed for single use and cannot be recharged. Once the battery is fully used, it must be replaced. - Voltage:
Lithium metal batteries typically provide a voltage of approximately 3.0V, higher than the 1.5V of other primary batteries such as alkaline batteries. - Long Shelf Life:
One of the most significant advantages of lithium metal batteries is their impressive shelf life, often lasting 10 to 15 years with proper storage. This makes them ideal for devices that require a reliable, long-term power source without frequent battery changes. - Low Self-Discharge Rate:
These batteries have a very low self-discharge rate, which means they lose very little energy when not in use. This contributes to their longevity.
Common Applications:
- Medical Devices: Pacemakers, hearing aids
- Consumer Electronics: Watches, calculators, remote controls
- Military and Aerospace: Valued for their high energy density and reliability in extreme conditions.
- Backup Systems: Memory backup for computers and other electronics
- Safety and Security Devices: Smoke detectors, emergency beacons
Safety Considerations:
- Low Thermal Stability: Lithium metal is highly reactive, which can lead to the risk of thermal runaway or fire if the battery is damaged or mishandled.
- Transportation Restrictions: Due to safety concerns, the transportation of lithium metal batteries, especially by air, is subject to regulations.
Lithium Metal Battery vs. Lithium-Ion Battery
The main difference between lithium metal batteries and lithium-ion batteries lies in their functionality and reusability. Lithium metal batteries are non-rechargeable and designed for single use, whereas lithium-ion batteries are rechargeable.
Both types of batteries share similar characteristics. Lithium metal batteries use metallic lithium as an electrode and generate electrical energy through the oxidation or corrosion of lithium. Once depleted, they cannot be recharged.
In contrast, lithium-ion batteries typically use lithium cobalt oxide as the positive electrode and carbon as the negative electrode. An electrolyte fills the space between the electrodes, allowing ions to flow. When charging, lithium ions move from the positive electrode to the negative electrode, embedding themselves in the carbon material. During discharge, the lithium ions return to the positive electrode.
Electrochemically, the key difference is that lithium-ion batteries use Li+ ions as charge carriers between electrodes, while lithium metal batteries use metallic lithium directly as the electrode material.
Part 3. Lithium Metal Batteries vs. Lithium-Ion Batteries
Lithium metal batteries and lithium-ion batteries are closely related, both relying on the migration of lithium ions (Li+) within the electrolyte during charging and discharging. In fact, lithium metal batteries can be considered a subset of lithium-ion batteries, where lithium metal is used as the electrode material. However, in most scientific literature, when we refer to “lithium-ion batteries,” we are usually referring to lithium-ion batteries that do not use lithium metal. When lithium metal is used as the electrode, it is more specifically referred to as a “lithium metal battery.”
Therefore, the distinction between lithium-ion batteries and lithium metal batteries becomes clear. In lithium-ion batteries, lithium exists only in the form of Li+ ions with a +1 charge, and there is no gain or loss of electrons during charging and discharging. In contrast, in lithium metal batteries, lithium undergoes a change in valence state during charging and discharging.
In some cases, the term “lithium battery” is used, which encompasses both lithium-ion batteries and lithium metal batteries. It is a generic term for any battery based on the element lithium.
Part 4. Summary
After decades of development, lithium-ion batteries have become a mature technology, widely used in daily life to power electronic devices and electric vehicles. In recent years, lithium metal batteries have attracted significant interest from researchers. Despite their potential, the widespread use of lithium metal batteries remains a challenge. Lithium metal is a reactive metal with high reactivity; it can react with water, oxygen, and other substances, and may burn in air, making it a hazardous material. Therefore, why do we continue to invest heavily in research on lithium metal batteries?
The main reason is the extremely high specific capacity of lithium metal batteries. Specific capacity refers to the number of electrons that can be gained or lost per unit mass of material. Compared to graphite anode material (C6Li), lithium metal has a specific capacity more than 11 times greater!
However, the commercialization of lithium metal batteries faces several obstacles, including the growth of lithium dendrites on the anode, the polysulfide shuttle effect in lithium-sulfur batteries, and safety concerns associated with lithium metal. However, we hope that continued research and development will overcome these challenges, leading to lighter electronic devices and electric vehicles with longer ranges in the future.
Part 5. Frequently Asked Questions
Can lithium metal batteries be recharged?
No, lithium metal batteries are primary batteries (non-rechargeable batteries). Recharging these batteries can lead to the formation of lithium dendrites. These dendrites can potentially cause short circuits and pose a significant safety risk.
What are the advantages of lithium-ion batteries?
Lithium-ion batteries have many advantages, including high energy density, low self-discharge rate, no memory effect, and the ability to be recharged multiple times. If you would like to learn more about lithium-ion battery life, please see our Golf Cart Battery Life Guide.
What are the safety concerns with lithium metal batteries?
Lithium metal batteries are prone to thermal runaway and explosion if damaged or overheated, as lithium metal is highly reactive and sensitive to temperature changes.
Can lithium-ion batteries be used in high-temperature environments?
Lithium-ion batteries have a limited operating temperature range, typically -20°C to 60°C (-4°F to 140°F). High-temperature environments can reduce battery performance and may create safety concerns.
What are some common applications of lithium metal batteries and lithium-ion batteries?
Lithium metal batteries are commonly used in small devices, such as watches, calculators, and hearing aids, where high energy density is crucial. On the other hand, lithium-ion batteries are widely used in portable electronics, electric vehicles, and energy storage systems due to their rechargeable nature and high energy density.

KHZH Articles
How to Safely Clean Leaking Battery Terminals: A Step-by-Step Guide
This comprehensive guide explains the risks involved, safety precautions, and proper techniques for cleaning terminals of leaking batteries, ensuring a safe and efficient process.
Portable Battery Charger vs. Power Bank: Understanding the Difference
A portable battery charger is a mobile charging device, while a power bank stores energy in its battery and can charge other devices without an external power source.
The Ultimate Guide to Using Lithium-Ion Jump Starters
Lithium-ion jump starters are critical tools in automotive emergencies. This guide covers how to safely use and maintain the jump starter, and explains why it’s an investment every driver should consider.
What is a Portable Battery Charger?
A portable battery charger is a convenient device that keeps your devices powered on the go. This guide clearly explains its function, materials, and operation, helping you stay connected wherever you are.
How to Choose the Best Battery Pack for Your Needs: Capacity, Performance, and More
Choosing the right battery pack is key to achieving reliable performance. This guide walks you through factors like capacity, safety, and performance to help you make an informed decision.