Lithium-containing batteries, such as those found in rechargeable devices such as cell phones, computers, and electric vehicles, play a crucial role in daily life. Simultaneously, research into lithium-based batteries remains a focal point of scientific innovation. Terms like “lithium battery,” “lithium-ion battery,” and “lithium-metal battery” are frequently used, but are they the same?
Both lithium-metal and lithium-ion batteries are types of lithium batteries. In this article, we will explore the differences between lithium-ion and lithium-metal batteries in detail.
Part 1: Exploring Lithium-Ion Batteries
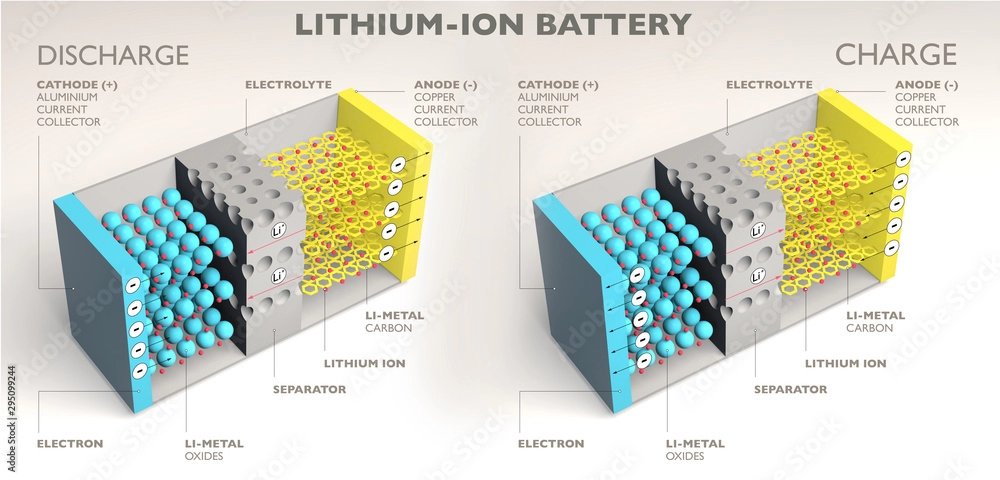
Lithium-ion batteries are a type of secondary (rechargeable) battery that primarily operates through the movement of lithium ions between the positive and negative electrodes. During charging and discharging cycles, lithium ions (Li+) move back and forth between the electrodes. When a lithium-ion battery is charged, Li+ ions are released from the positive electrode and embedded into the negative electrode through the electrolyte, enriching the negative electrode with lithium ions. Conversely, during discharge, this process is reversed.
1. Components of a Lithium-Ion Battery
A. Positive Electrode (Cathode) (正极):
Commonly used active materials for the positive electrode include lithium manganese oxide, lithium cobalt oxide, and lithium nickel manganese cobalt oxide (often referred to as ternary material). In applications like electric bicycles, a combination of lithium nickel manganese cobalt oxide, or ternary material, with a small amount of lithium manganese oxide is often preferred. Pure lithium manganese oxide and lithium iron phosphate are less commonly used due to their bulkier size, lower performance, or higher cost. The conductive current collector used in the positive electrode is typically aluminum foil with a thickness of 10-20 micrometers.
B. Separator:
The separator is a specially designed polymer film with a microporous structure. It allows lithium ions to pass through easily while blocking electrons, preventing short circuits.
C. Negative Electrode (Anode) (负极):
The active material for the negative electrode is typically graphite or graphite-based compounds. The conductive current collector for the negative electrode is copper foil, with a thickness ranging from 7 to 15 micrometers.
D. Organic Electrolyte:
Organic electrolytes typically consist of carbonate-based solvents with lithium hexafluorophosphate dissolved in them. Lithium polymer batteries use a gel electrolyte, which functions similarly.
E. Battery Case:
Lithium-ion batteries come in different cases, including steel cases (less common for square shapes), aluminum cases, nickel-plated iron cases (commonly used for cylindrical cells), and aluminum-plastic laminate (used for pouch cells). The battery cap also serves as the positive and negative terminals.
2. How Lithium-Ion Batteries Work
Lithium-ion batteries use carbon-based materials as the negative electrode and lithium-containing compounds as the positive electrode. Notably, no lithium metal is involved; only lithium ions are present. This is a key characteristic of lithium-ion batteries.
Lithium-ion batteries utilize lithium-ion intercalation compounds as the active material in the positive electrode. The charging and discharging processes primarily involve the intercalation and deintercalation of lithium ions between the two electrodes. This process is often described as the insertion and extraction of lithium ions. As lithium ions move between the electrodes, electrons are also intercalated and deintercalated. This process gives rise to the “rocking chair” analogy for these batteries, as the lithium ions ‘rock’ back and forth between the electrodes.
When a lithium-ion battery is charged, lithium ions are generated at the positive electrode. These ions then move through the electrolyte to the negative electrode. The negative electrode is made of carbon, which has a layered structure with many micropores. Lithium ions are inserted into these micropores, and the number of lithium ions inserted determines the battery’s state of charge.
Stay tuned for our upcoming section on lithium-metal batteries, where we’ll explore the key differences between these two important battery technologies! For those interested in different battery types and their applications, check out our LiFePO4 vs Li-ion vs Li-Po Battery Complete Guide.
Overview of Lithium-Ion Batteries
1. How Lithium-Ion Batteries Work
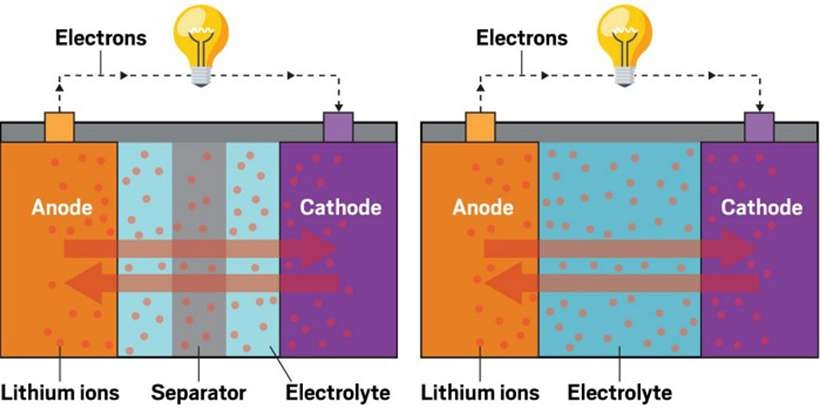
Lithium-ion batteries work by moving lithium ions between two electrodes—a positive electrode (cathode) and a negative electrode (anode)—and moving through an electrolyte. When charging, lithium ions are embedded in the carbon layers of the anode. Conversely, during discharge, these ions are released from the anode and return to the cathode, powering the battery.
The discharge capacity of a battery depends on how many lithium ions are able to return to the cathode, which directly affects its performance.
Typically, the charging current for lithium batteries is set between 0.2C and 1C. Higher currents allow for faster charging but also generate more heat. Using too high a current during charging can prevent the battery from fully charging because the electrochemical reactions within the battery require time. This can be likened to pouring a beer—if poured too quickly, it will foam and overflow, resulting in the battery not fully charging.
2. Advantages of Lithium-Ion Batteries
A. High Voltage
A single lithium-ion battery typically operates at 3.7–3.8V (compared to 3.2V for lithium iron phosphate batteries), which is approximately three times the voltage of nickel-cadmium and nickel-metal hydride batteries.
B. Specific Capacity
Lithium-ion batteries have a specific capacity of approximately 555Wh/kg, which is about 3-4 times that of nickel-cadmium batteries and 2-3 times that of nickel-metal hydride batteries. The actual capacity is approximately 88% of its theoretical maximum.

Lithium Metal Batteries: High-Energy, Long-Life, and Reliable Power Source
Lithium metal batteries are a type of non-rechargeable (primary) battery that uses pure metallic lithium as an anode. These batteries are known for their impressive energy density and long lifespan, making them ideal for applications that require a reliable power source in a compact design.
Key Features of Lithium Metal Batteries:
High Energy Density:
Lithium metal batteries offer a higher energy density compared to other types of batteries, such as alkaline or zinc batteries. This allows them to store more energy in a smaller and lighter package, making them ideal for portable devices that require a sustained power supply.
Non-Rechargeable:
As primary batteries, lithium metal batteries are designed for single use and cannot be recharged. Once the battery is depleted, it must be replaced, ensuring a fresh and reliable power source when needed.
Voltage:
Lithium metal batteries typically operate at a voltage of around 3.0V, which is significantly higher than most other disposable batteries, such as alkaline batteries (typically 1.5V). This higher voltage enhances their performance, making them suitable for high-power devices.
Long Shelf Life:
Lithium metal batteries are designed to have a long shelf life, often lasting 10-15 years under proper storage conditions. This makes them well-suited for applications where long-term power supply is needed without frequent battery replacements.
Low Self-Discharge Rate:
One notable feature is their extremely low self-discharge rate, contributing to their extended operational life and reliability.
Common Applications of Lithium Metal Batteries:
- Medical Devices: Pacemakers, hearing aids
- Consumer Electronics: Watches, calculators, remote controls
- Military and Aerospace: Widely used in military and aerospace applications due to their high energy density and reliability in extreme conditions
- Backup Systems: Memory backup for computers and other electronic devices
- Safety and Security Equipment: Smoke detectors, emergency beacons
Safety Considerations:
Poor Thermal Stability:
Metallic lithium is highly reactive and can lead to thermal runaway or fire if the battery is damaged or mishandled. Handle and dispose of these batteries with care.
Transportation Restrictions:
Due to safety concerns, lithium metal batteries are subject to transportation restrictions, especially for air transport, to reduce the risk of incidents during transit.
Lithium Metal vs. Lithium-Ion Batteries
The primary distinction between lithium metal and lithium-ion batteries is that lithium metal batteries are disposable, whereas lithium-ion batteries are rechargeable. Both battery types utilize lithium, but their mechanisms for generating electrical energy differ.
Lithium Metal Batteries:
Lithium metal batteries use metallic lithium as an electrode, generating electrical energy through the oxidation of metallic lithium. Once the battery is used up, it cannot be recharged and must be replaced, which is typical for most disposable (primary) batteries.
Lithium-Ion Batteries:
Lithium-ion batteries, on the other hand, are designed to be rechargeable. They typically use a lithium cobalt oxide compound as the positive electrode and carbon as the negative electrode. The electrolyte in the battery facilitates the movement of lithium ions between the positive and negative electrodes. During charging, lithium ions move from the positive electrode to the negative electrode, and during discharge, they flow back to the positive electrode. This continuous movement of lithium ions enables the battery to be recharged multiple times.
Electrochemical Differences:
The key difference between lithium metal and lithium-ion batteries lies in their use of lithium. Lithium-ion batteries use lithium ions (Li+) to transfer energy between the electrodes, while lithium metal batteries use pure metallic lithium as the anode. This difference in electrode materials is what makes lithium-ion batteries rechargeable, while lithium metal batteries are not.
In summary, while both battery types utilize lithium, their composition and the methods by which they store and transfer energy set them apart, offering various benefits depending on the intended application.
KHZH is dedicated to providing reliable, high-performance energy solutions, powering your devices.
Part 1: Lithium Metal Batteries vs. Lithium-ion Batteries
Lithium metal batteries involve the migration of Li+ ions in the electrolyte during charging and discharging. Technically, lithium metal batteries are one of the broader categories of lithium batteries; in fact, lithium batteries represent a broader category that includes lithium metal batteries. However, in scientific literature, the term “lithium-ion battery” commonly refers to lithium-ion batteries that do not use a lithium metal electrode. When lithium metal is used as the electrode, the battery is typically referred to as a “lithium metal battery.”
With this definition in mind, the distinction between the two becomes clearer. In lithium-ion batteries, lithium exists only in the form of Li+ ions, meaning that no gain or loss of electrons occurs during charging and discharging cycles. On the other hand, in lithium metal batteries, lithium undergoes changes in its oxidation state during charging and discharging.
The term “lithium battery” has also emerged, which is a broader category that includes both lithium metal batteries and lithium-ion batteries. It serves as a collective term for batteries that utilize lithium-based elements.
Part 4: Summary
Lithium-ion batteries, after decades of development, are now a mature and widely used technology in various devices. These batteries power everyday items such as electronics and electric vehicles. However, lithium metal batteries have been a major focus of scientific research in recent years. Despite progress, they still face challenges before becoming commercially viable.
Lithium metal, as an alkali metal, has reactive chemical properties. It can react with water, oxygen, and other substances, and may even burn in air, making it a hazardous material. Despite these challenges, why continue to invest heavily in lithium metal battery research and development?
The main reason is the impressive specific capacity of lithium metal batteries. Specific capacity refers to the amount of charge that a material can store per unit mass. In comparison, the charge storage capacity of a lithium metal anode is more than 11 times that of a graphite lithium compound (C6Li) anode!
However, commercialization is hindered by issues such as the formation of dendrites on the lithium metal anode, the polysulfide shuttle effect in lithium-sulfur batteries, and safety concerns associated with lithium metal. These challenges also impact other promising battery technologies, such as lithium-sulfur batteries, which suffer from the polysulfide shuttle effect. Researchers are actively working to overcome these challenges, leading to lighter electronic devices and longer-lasting electric vehicles.
Part 5: Frequently Asked Questions (FAQ)
Are lithium metal batteries rechargeable?
Currently, lithium metal batteries are typically used as primary (non-rechargeable) batteries. Charging them can lead to the formation of lithium dendrites, which can cause short circuits and safety hazards.
What are the advantages of lithium-ion batteries?
Lithium-ion batteries have several advantages, including high energy density, low self-discharge rate, no memory effect, and the ability to be recharged multiple times. If you are interested in learning more about the different types of lithium batteries, check out our guide, LFP (Lithium Iron Phosphate) Battery vs Lithium Ion.
What are the safety concerns with lithium metal batteries?
The reactivity of metallic lithium makes lithium metal batteries prone to thermal runaway and explosions if damaged or overheated.
Can lithium-ion batteries be used in high-temperature environments?
Lithium-ion batteries have a limited safe operating temperature range, typically between -20°C and 60°C (-4°F and 140°F). Operating outside this range can reduce battery performance and pose safety risks.
What are the common applications of lithium metal and lithium-ion batteries?
Lithium metal batteries are commonly used in small devices such as watches and calculators, where high energy density is required. Lithium-ion batteries are widely used in portable electronics, electric vehicles, and energy storage systems due to their rechargeable nature and high energy density.

KHZH – Electrical Engineering Insights
More Great Articles
How to Safely Clean Battery Terminals Affected by Leakage (Posts): A Step-by-Step Guide
This comprehensive guide details the risks, safety precautions, and proper cleaning methods for handling battery terminals affected by leakage. We’ll walk you through the steps.
Portable Battery Charger vs. Power Bank: Understanding the Difference
A portable battery charger is a generic term for various mobile charging devices, while a power bank has a built-in battery that can charge devices without an external power source.
The Ultimate Guide to Lithium-Ion Jump Starters
Lithium-ion jump starters are valuable tools in emergency automotive situations. This guide covers their usage, safety tips, maintenance, and explains why they’re a smart investment for any car owner.
What is a Portable Battery Charger?
A portable battery charger is an essential gadget for ensuring your devices stay powered on the go. This guide details in simple terms what it is, the materials it’s made of, and how it works.
How to Choose the Best Battery Pack for Your Needs: Capacity, Performance, etc.
Selecting the right battery pack is crucial for ensuring reliable performance for your device. This guide highlights key factors, including capacity and safety, to help you make an informed decision.