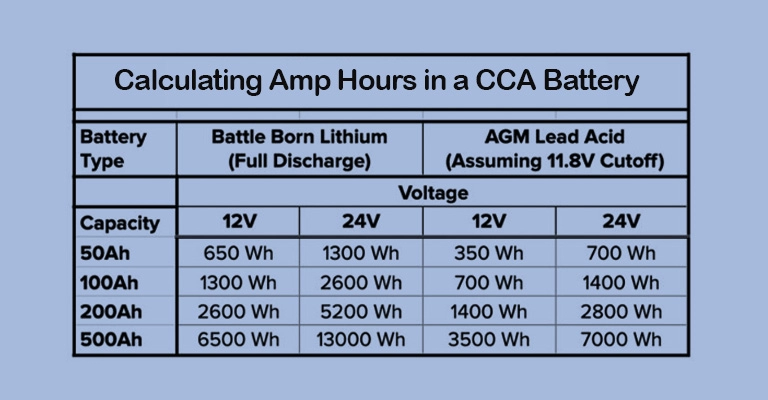
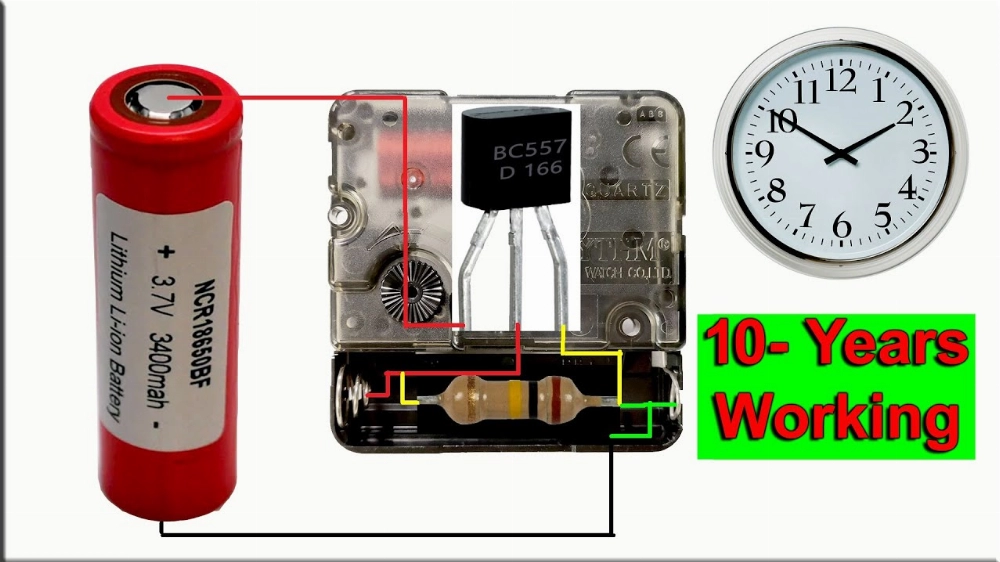
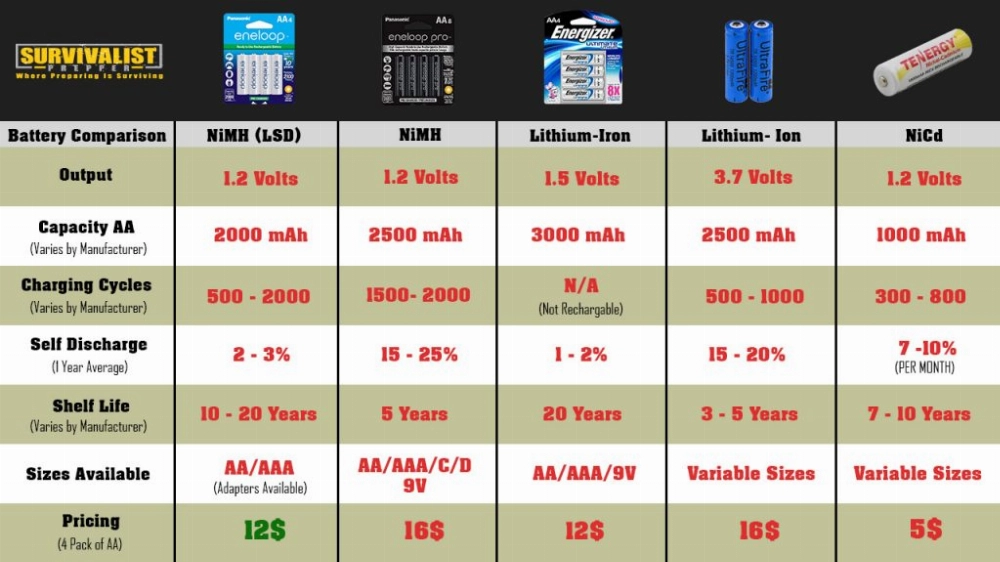
Part 1. What are Atomic Batteries?
How Does an Atomic Battery (Nuclear Battery) Work?
An atomic battery, also known as a nuclear battery or radioisotope thermoelectric generator (RTG), generates electricity from the energy released by the decay of a radioactive isotope. Unlike traditional batteries that rely on chemical reactions, atomic batteries convert the heat produced during radioactive decay into electrical energy. This process is accomplished using a thermoelectric material that leverages the Seebeck effect, generating an electric current when exposed to a temperature difference.
Atomic batteries are unique because they can last for many years, even decades, without requiring replacement or recharging. This durability makes them indispensable in fields where frequent replacement or recharging is difficult or impossible, such as deep-space exploration, remote scientific research stations, and medical implants.
Part 2. Who Invented the Atomic Battery?
The concept of the atomic battery dates back to the early 20th century. In 1913, English physicist Henry Moseley first proposed the idea of using the energy produced by radioactive decay to generate electricity. However, it wasn’t until the 1950s that practical atomic batteries began to emerge.
During the Cold War, the U.S. Atomic Energy Commission (AEC) began focusing on the peaceful uses of nuclear energy. It was during this period that the first functional atomic batteries were developed for military equipment and spacecraft. The U.S. space program, including NASA, found atomic batteries particularly useful for providing long-term, reliable power in environments where solar energy was not feasible.
The development of the radioisotope thermoelectric generator (RTG) was a key breakthrough, and it became the foundation for many space missions. The Voyager spacecraft, launched in 1977, and is still operating today. It stands as a prime example of a space mission powered by RTGs for over four decades.
Part 3. Atomic Battery vs. Lithium-Ion, NiMH, and Lead-Acid Batteries
Atomic batteries, like other modern battery technologies, offer application scenarios suited to specific needs. Here’s a comprehensive comparison between atomic batteries and popular commercial battery types, including lithium-ion, nickel-metal hydride (NiMH), and lead-acid batteries.
1. Energy Density and Longevity
-
Atomic Batteries:
Atomic batteries have exceptionally high energy density. Because they rely on radioactive decay, they can operate continuously for decades without requiring recharging. This makes them ideal for long-term, maintenance-free applications, such as space missions and remote scientific instruments. -
Lithium-Ion Batteries:
Lithium-ion batteries offer high energy density, and are rechargeable, making them ideal for consumer electronics, electric vehicles, and renewable energy storage. However, they degrade over time and typically have a lifespan of only a few years. For more on optimizing lithium battery use, check out our battery storage solutions. -
Nickel-Metal Hydride (NiMH) Batteries:
NiMH batteries offer moderate energy density, and are rechargeable. They have a shorter lifespan than lithium-ion batteries and are commonly used in hybrid vehicles and some household electronics. -
Lead-Acid Batteries:
Lead-acid batteries have relatively low energy density, and a shorter lifespan. However, they are cost-effective, making them widely used in automotive starters, backup power systems, and industrial applications.
Winner: Atomic batteries lead in energy density and longevity, but their radioactivity makes them unsuitable for general-purpose use.
2. Power Output
-
Atomic Batteries:
These batteries provide a consistent, low power output over extended periods, which makes them unsuitable for applications requiring high, instantaneous energy demands, such as electric vehicles or heavy machinery. -
Lithium-Ion Batteries:
Lithium-ion batteries are particularly well-suited for delivering high power output in applications requiring quick bursts of energy. -
Nickel-Metal Hydride (NiMH) Batteries:
NiMH batteries offer moderate power output and are often used in applications requiring sustained power, but not high power demands. -
Lead-Acid Batteries:
Lead-acid batteries provide a stable but lower power output and are commonly used in automobiles and backup systems where higher power bursts are not needed.
Winner: Lithium-ion batteries excel in power output for high-energy-demand applications.
Atomic Batteries vs. Lithium-Ion, NiMH, and Lead-Acid Batteries: A Comparative Analysis
When considering energy storage options for various applications, it is crucial to evaluate the characteristics of different battery technologies. In this comparison, we will examine several key properties of atomic batteries, lithium-ion batteries, nickel-metal hydride (NiMH) batteries, and lead-acid batteries.
1. Energy Density
Atomic Batteries
Atomic batteries boast extremely high energy density due to the use of radioactive materials. This allows them to produce a significant amount of energy over extended periods with minimal weight, making them suitable for specialized applications like space missions and remote sensors.
Lithium-Ion Batteries
Lithium-ion batteries offer high energy density, making them ideal for consumer electronics, electric vehicles (EVs), and renewable energy systems. Their performance continues to improve, positioning them as a leading technology in modern energy storage.
NiMH Batteries
NiMH batteries have a moderate energy density and are commonly used in hybrid vehicles and household devices. While not as energy-dense as lithium-ion batteries, they serve as an alternative when lithium-ion technology is not feasible.
Lead-Acid Batteries
Lead-acid batteries have the lowest energy density compared to other technologies, but their high surge current makes them suitable for applications like automotive starters and backup power systems.
Winner: Atomic batteries lead in energy density, followed by lithium-ion batteries. NiMH and lead-acid batteries have lower energy densities.
2. Lifespan
Atomic Batteries
One of the most notable features of atomic batteries is their exceptionally long lifespan. They can last from 10 to 100 years, providing reliable power for extended durations without the need for replacement. This makes them well-suited for applications where maintenance is challenging, such as space probes and pacemakers.
Lithium-Ion Batteries
Lithium-ion batteries typically last for 2-5 years, which is long compared to *older* battery technologies. However, their lifespan depends on factors such as usage, charge cycles, and temperature conditions.
NiMH Batteries
NiMH batteries have a lifespan of about 2-3 years, shorter than lithium-ion batteries, but still sufficient for most hybrid vehicle applications and some household electronics.
Lead-Acid Batteries
Lead-acid batteries have the shortest lifespan of the group, typically lasting 1-3 years, which can be a limitation for long-term energy storage solutions.
Winner: Atomic batteries stand out with unmatched longevity, while lithium-ion batteries offer a good balance of lifespan for most modern applications.
3. Power Output
Atomic Batteries
Atomic batteries offer low power output, making them unsuitable for high-power applications. However, their extremely long lifespan and ability to operate in extreme conditions make them ideal for remote devices requiring minimal maintenance.
Lithium-Ion Batteries
Lithium-ion batteries provide high power output, which is essential for powering devices like smartphones, laptops, and electric vehicles. Their versatility and adaptability to both high and low-power demands make them the most widely used power source.
NiMH Batteries
NiMH batteries offer moderate power output, making them suitable for hybrid vehicles and low-power consumer electronics. They cannot match the performance of lithium-ion batteries but offer a more affordable alternative.
Lead-Acid Batteries
Lead-acid batteries provide high surge current, making them ideal for applications like automotive starters and uninterruptible power supplies (UPS) (also known as UPS). While they can meet high-power demands, they sacrifice energy density.
4. Environmental Impact
Atomic Batteries
Atomic batteries use radioactive materials, which raise significant environmental concerns regarding disposal and potential contamination. Strict handling and regulatory measures must be in place to manage the risks associated with these batteries.
Lithium-Ion Batteries
Lithium-ion batteries present challenges due to their reliance on limited resources like lithium and cobalt. Improper disposal of these batteries can lead to environmental hazards, although recycling programs are steadily improving.
NiMH Batteries
NiMH batteries are considered less environmentally harmful than lithium-ion batteries, as they do not contain heavy metals like cobalt. However, their production process still has environmental impacts, and recycling technologies are not as mature as those for lithium-ion batteries.
Lead-Acid Batteries
Lead-acid batteries are highly recyclable, but they pose significant environmental risks if not disposed of properly. The toxic lead must be handled carefully to prevent contamination.
Winner: NiMH batteries have a relatively lower environmental impact compared to lithium-ion and lead-acid batteries, but no technology is entirely free of environmental concerns.
5. Cost
Atomic Batteries
Atomic batteries are expensive to produce due to the specialized technology and radioactive materials involved. Their high cost limits their use to specialized applications, such as space exploration.
Lithium-Ion Batteries
Lithium-ion batteries have become more affordable over the years due to advancements in manufacturing technology and economies of scale. However, they are still more expensive than older technologies like lead-acid batteries.
NiMH Batteries
NiMH batteries are moderately priced and typically cheaper than lithium-ion batteries. However, they are being phased out in favor of lithium-ion technology for many applications.
Lead-Acid Batteries
Lead-acid batteries are the most cost-effective option, making them popular in cost-sensitive applications like automotive starters and backup power systems.
Winner: Lead-acid batteries are the cheapest, while atomic batteries are the most expensive.
6. Applications
Atomic Batteries
Atomic batteries are well-suited for long-term, low-maintenance applications, particularly in extreme environments. They have been used in space probes, pacemakers (historically), and remote sensors that require decades of continuous power.
Lithium-Ion Batteries
Lithium-ion batteries dominate the consumer electronics, electric vehicle, and renewable energy system markets due to their high energy density and rechargeability.
NiMH Batteries
NiMH batteries are commonly used in hybrid vehicles and some household electronics. However, they are increasingly being replaced by lithium-ion batteries as technology advances.
Lead-Acid Batteries
Lead-acid batteries are primarily used in automotive starters, uninterruptible power supplies (UPS), and large-scale energy storage.
Winner: Lithium-ion batteries are the most versatile, although each battery type excels in its own specialized area.
Summary Table
| Feature | Atomic Batteries | Lithium-Ion Batteries | NiMH Batteries | Lead-Acid Batteries |
|---|---|---|---|---|
| Energy Density | Extremely High | High | Moderate | Low |
| Lifespan | Decades | 2-5 Years | 2-3 Years | 1-3 Years |
| Power Output | Low | High | Moderate | High |
| Environmental Impact | High (Radioactive Waste) | Moderate (Requires Recycling) | Lower than Lithium-Ion | High (Toxic Lead) |
| Cost | Very High | Moderate | Moderate | Low |
| Applications | Niche | Versatile | Limited | Cost-Sensitive |
A Complete History of Lithium-Ion Batteries
Part 4: Advantages and Disadvantages of Atomic Batteries
Atomic batteries have several notable advantages, especially in terms of lifespan. These batteries can last from 10 to 100 years, making them ideal for long-term, low-maintenance power solutions in extreme environments. However, the reliance on radioactive materials also presents significant challenges in terms of safety and waste disposal.
Pros and Cons of Atomic Batteries
Advantages:
- Durable Power Source: Atomic batteries utilize the stable decay of isotopes, making them highly suitable for long-term power needs in applications like space missions and medical devices.
- Stable Power Output: Unlike chemical batteries that degrade over time, atomic batteries offer a stable energy output until the radioactive material is fully depleted.
- Minimal Maintenance: Due to the absence of moving parts and their reliance on natural radioactive decay, atomic batteries are exceptionally durable and require minimal maintenance. This is particularly useful in remote or hard-to-reach locations.
- High Energy Density: Atomic batteries have a higher energy density compared to traditional batteries, allowing them to store more energy in a smaller space.
- Small Footprint: Despite their significant energy capacity, atomic batteries are often small and lightweight, making them ideal for applications where space is limited.
Disadvantages:
- Radioactivity: The use of radioactive materials presents significant safety concerns. While atomic batteries are designed with safety in mind, improper handling or disposal can still lead to radiation exposure.
- High Cost: The development and manufacturing of atomic batteries are expensive due to the specialized materials and advanced technology required. This makes them unsuitable for everyday consumer products.
- Limited Applications: Due to their high cost and the involvement of radioactive materials, atomic batteries are primarily used in specialized fields such as space exploration, military, and specific medical devices.
- Environmental Impact: While generally safe when used correctly, the long-term environmental impact of atomic batteries, particularly concerning the disposal of radioactive materials, remains a concern.
The Role of Atomic Batteries in Modern Society
Atomic batteries, or nuclear batteries, are a specialized type of power source that generates electricity from radioactive decay. While not commonly used for everyday purposes, they have made a significant impact in several niche areas. The following sections detail the roles atomic batteries have played:
1. Advancements in Space Exploration
- Reliable Power in Extreme Environments: Atomic batteries play a crucial role in space exploration, powering spacecraft, rovers, and satellites in environments where solar power is not effective, such as deep space, the far side of the Moon and shadowed regions of Mars.
- Example: NASA’s Voyager probes, launched in 1977, are still operational today, thanks to their Radioisotope Thermoelectric Generators (RTGs).
- Longevity: The decades-long lifespan of atomic batteries ensures continuous operation for deep-space missions.
2. Medical Applications
- Pacemakers and Medical Devices: Atomic batteries were once used in pacemakers to provide a reliable and long-lasting power source. While modern pacemakers now use rechargeable lithium-ion batteries, early nuclear-powered pacemakers significantly improved the lifespan and reliability of these devices.
- Radiopharmaceutical Development: Principles of nuclear energy have also advanced the application of nuclear medicine in diagnostics and treatments.
3. Military and Defense Applications
- Remote Monitoring and Surveillance: Atomic batteries power remote sensors, underwater detection systems, and surveillance equipment that require long-term, maintenance-free power.
- Miniaturized Electronics: The development of atomic battery technology has spurred innovation in compact power solutions for critical military applications.
4. Scientific Research
- Remote Scientific Stations: Atomic batteries are used to power instruments in remote and extreme environments, such as polar research stations and deep-sea exploration equipment.
- Data Collection: They are also used in monitoring systems, such as seismic sensors, to collect long-term environmental and geological data.
5. Energy Technology
Atomic batteries, with their high energy density and long lifespan, indirectly contribute to energy technology advancements, offering valuable insights for improving energy solutions in various industries.
Atomic Battery Innovations
The Inspiration Behind Battery Development:
While not directly used in consumer electronics, atomic batteries have significantly advanced research toward more durable and efficient energy storage solutions, particularly in extreme or specialized environments.
Potential Future Applications:
As the demand for sustainable, long-lasting energy grows, atomic batteries could become a key component in future renewable energy systems or microgrid technologies. For example, while not atomic, lithium batteries have been widely adopted as reliable energy storage systems in various applications.
Societal Considerations
Safety Concerns:
The use of radioactive materials in atomic batteries raises significant safety and environmental concerns, which currently limit their wider application.
Ethical and Regulatory Challenges:
Strict regulations govern the use and disposal of radioactive materials, ensuring these technologies are developed and used in a responsible manner.
Section 6: Recent Advances in Atomic Battery Technology
Recent advances in atomic battery development have focused primarily on increasing efficiency while minimizing safety risks. Current research aims to identify superior thermoelectric materials for more efficient conversion of heat to electricity. Scientists are also studying Betavoltaic batteries, which harness energy from beta particles released by radioactive decay to generate electricity. These batteries are projected to be smaller and safer than traditional Radioisotope Thermoelectric Generators (RTGs), unlocking potential applications in medical devices, sensors, and other compact technologies.
Related Topics:
-
Understanding 11.1V LiPo Batteries: Characteristics, Advantages, and Common Applications
11.1V LiPo batteries are commonly used to power remote-controlled models, drones, etc. This guide discusses their characteristics, advantages, and common applications. -
Lithium-Ion Jump Starter vs. Lead-Acid Battery: What’s the Real Difference?
Lithium-ion jump starters offer a quick and reliable way to jump-start a dead car battery. Here’s a comparison with traditional lead-acid models. -
How to Safely Clean Battery Leakage from Terminals/Contacts: A Step-by-Step Guide
This guide provides essential safety tips and methods for cleaning battery leakage from terminals/contacts. -
Portable Charger vs. Power Bank: What’s the Difference?
While both are portable charging solutions, a portable charger charges devices directly, while a power bank stores energy for later use without an external power source. -
The Ultimate Guide to Using a Lithium-Ion Jump Starter:
A lithium-ion jump starter is a wise investment for automotive emergencies. This guide covers its usage, safety, maintenance, and benefits.