This glossary of technical terms is designed to help you understand standard terms used in the battery industry.
Active Materials
Active electrochemical materials are used in the manufacture of positive and negative electrodes.
Absorbent Glass Mat (AGM)
Absorbent Glass Mat (AGM) is a type of lead-acid battery that uses glass mats to facilitate the recombination of gases produced during the charging process.
Ambient Temperature
The prevailing surface temperature at which a battery is located.
Ampere
The unit of measurement for current.
Ampere-hour
The product of current (amps) times time (hours). Used to express the capacity of a battery, also known as Amp. or A.H.
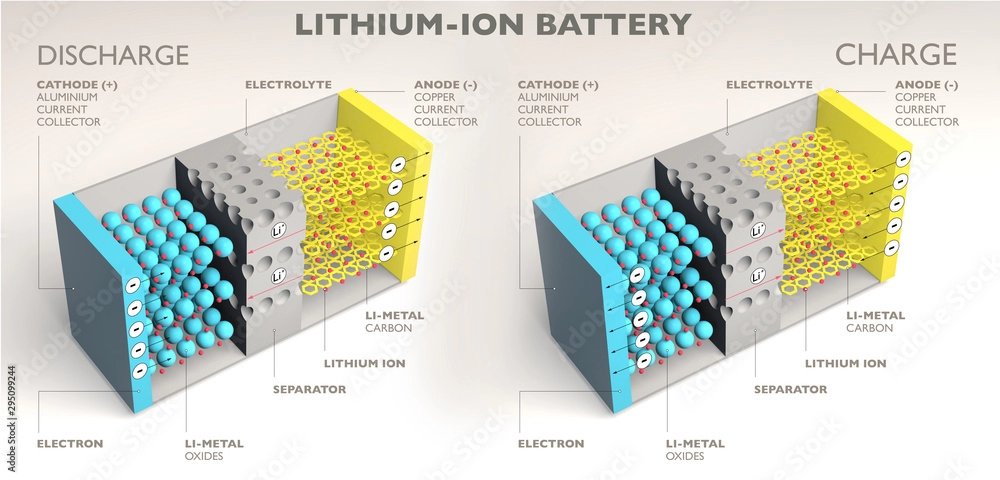
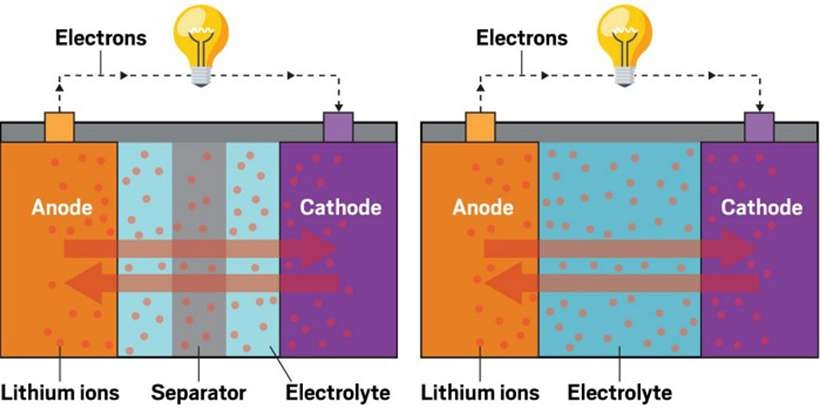
Anode.
The electrode releases electrons when discharged. When supplying power to a device, the anode is positive; when discharging, the anode becomes negative.
Battery
Two or more cells are connected in series or parallel.
Battery Energy Storage System
Battery Energy Storage System, sometimes called ESS or BESS.
BMS Battery Management System
A battery management system is used inside or outside a battery to manage charging and discharging and provide SoC and SoH data. It is used to protect and maximize the life of the battery.
Bluetooth
Low-power radio communication within 10 meters (30 feet). Power-Sonic Bluetooth Lithium batteries use Bluetooth with the BMS to provide instant access to battery status from smart devices.
C
indicates a charge or discharge rate equal to the battery capacity divided by one hour. Thus, a 2000 mAh battery has a C value of 2.0 A, an identical battery has a C/5 value of 400 mA, and a C/10 value of 200 mA.
Capacity
The usable electrical energy of a battery or battery pack is expressed in ampere-hours.
- Available Capacity: The number of ampere-hours a battery can discharge based on its state of charge, discharge rate, ambient temperature, and specified cutoff voltage.
- Rated Capacity (“C”): The discharge capacity specified by the manufacturer as available at a specific discharge rate and temperature.
- Capacity Decay: Loss of capacity due to undercharging.
- Capacity Offset: The capacity correction when a battery is discharged at a higher C rate than specified.
Cathode
The electrode in a battery reduces by absorbing electrons. When discharging, the cathode is positive, and the opposite is true when charging, becoming negative.
Cell
The essential component of a battery. Lead-acid batteries have a nominal voltage of 2 volts, and lithium iron phosphate batteries have a nominal voltage of 3.2 volts.
Secondary Battery: The process is reversible and can be charged and discharged repeatedly.
- Battery Reversal: The act of reversing the polarity of a battery by over-discharging it.
- Mismatched Cells: Cells in a battery pack are not equal in capacity, voltage, or internal resistance.
- Primary Battery: A battery or battery pack that can only be discharged once and cannot be recharged. Examples include alkaline manganese-zinc batteries.
- Secondary Battery: A battery in which the process is reversible so that it can be charged and discharged repeatedly. Examples include lead-acid batteries.
- Cylindrical batteries: The positive and negative plates are rolled up and placed in a cylindrical container. Examples include AA batteries and 18650 batteries.
- Prismatic Batteries: Batteries in which the positive and negative plates are stacked together rather than rolled.
- Pouch Battery: Packaged in a flexible, heat-sealed aluminum foil pouch.
- Power Cells: Batteries designed to deliver maximum current and high discharge rates.
- Energy Batteries: Batteries designed for maximum capacity. Longer cycle life.
Rechargeable
The process of converting electrical energy into chemical energy and restoring electrical power to a cell or battery.
- BATTERY HOLDING POWER: The ability of a battery to hold a charge. It diminishes during storage.
- CHARGE ACCEPTANCE: Quantifies the amount of charge accumulated in a battery.
- FLOAT CHARGE: Maintains the capacity of a battery or battery pack by applying a constant voltage.
- Trickle Charge: Maintains the capacity of a cell or battery pack by applying a small constant current.
- Equalize Charge: To bring all cells in a battery pack or string to the same state of charge.
Closed Circuit Voltage Test
A test method in which a battery is briefly discharged at a constant current while the voltage is being measured.
Coulomb
A unit of electrical charge. One coulomb (1C) equals one-ampere second (1A).
Cutoff Voltage
The final voltage of a cell or battery at the end of charging or discharging.
Cycle
A single charge or discharge of a cell or battery.
- Cycle Life: The total number of cycles before a battery reaches the end of its life.
Deep Cycle
A cycle in which a battery is continuously discharged until it reaches its cut-off voltage, usually 80% of the discharge of a lead-acid battery.
Direct Current (DC)
The type of current a battery can deliver. One terminal is always positive, and the other terminal is always negative.
Discharge
The process of drawing current from a battery.
- Deep Discharge: The discharge of a cell or battery to between 80% and 100% of its rated capacity.
- Depth of Discharge (DoD): The capacity lost during discharge, usually expressed as a percentage.
- Self-discharge: The loss of capacity when a battery is stored or not in use.
- Self-Discharge Rate: Percentage of capacity lost when the circuit is open for a given period.
Depletion.
The amount of current drawn from a battery.
- Parasitic Discharge: Continued loss of battery power after the vehicle engine is turned off.
Electrodes
Positive or negative plates contain material that reacts with an electrolyte to produce or receive an electric current.
Electrolyte.
The conductive ions in a battery. Lead-acid batteries use a sulfuric acid solution.
End-of-Charge Voltage
The voltage reached by a cell or battery pack at the end of a charge while the charger is still connected to the battery.
Energy Density
The ratio of a battery’s energy to its volume or weight in watt-hours/cubic inches/mm or pounds/kg.
Gas Reorganization
The process by which oxygen produced by the positive plates during the final stages of charging is absorbed by the negative plates to prevent water loss.
Rapid Discharge
The rapid discharge of a battery. Usually, a multiple of C (battery rating expressed in amperes).
Specific Gravity Meter
A device used to measure the specific gravity of a liquid to read the state of charge of a flooded lead-acid battery.
Impedance
The resistance of a battery to alternating current in ohms (Ω). Usually measured at 1000 Hz at full charge.
Internal Resistance
The resistance within a battery produces a voltage drop proportional to the current.
Lithium-ion Battery
A rechargeable battery with cobalt, manganese, iron, and other metals as the cathode and graphite as the anode.
Negative Electrode
The cell end of a battery where electrons flow in an external circuit when the battery is discharged.
Nominal Capacity
The nominal value of the rated capacity.
Rated Voltage
The nominal value of the rated voltage.
Open Circuit Voltage
The voltage of a battery or cell is measured in the unloaded state.
Overcharge
Continuing to charge a battery after it has reached 100% capacity. Prolonged overcharging will shorten battery life.
Parallel connection
Connect a group of cells or batteries by connecting all terminals with the same polarity. This increases the capacity of the battery pack.
Polarity
The charge on the battery terminals.
Positive
The battery terminal to which electrons flow through the external circuit when the battery is discharged.
Rated Capacity
The capacity of a battery is measured in amperes. Usually, the specified depth of discharge is at room temperature for a specified number of hours at a constant current.
Recombination
The state where the gases typically formed during battery operation recombine to form water.
Series Connection
Connecting a group of cells or batteries by connecting terminals of opposite polarity. This increases the voltage of the battery pack.
Self-Discharge
The battery’s capacity is lost in a stored or unused condition without external discharge.
Separator
The material that separates the positive and negative plates. In sealed lead-acid batteries, absorbent glass fibers often suspend the electrolyte.
SLA Battery
A sealed lead-acid battery is usually characterized by Maintenance-free and leak-proof features. This type of battery has a safety vent that releases gas in case of high internal pressure. It is, therefore, also known as a valve-regulated battery or VRLA.
Gel batteries are a type of SLA battery in which the dilute sulfuric acid electrolyte is immobilized by additives that turn the electrolyte into a gel.
Service life
The expected service life of a battery is expressed in terms of the total number of cycles or years of standby service and a specified percentage of the original capacity remaining.
Shelf life
The maximum time a battery can be stored without recharging.
Standby Service
An application in which trickle or float charging keeps the battery fully charged.
State of Charge (SoC)
The available capacity of a battery for a given period is expressed as a percentage of the rated capacity.
Absolute State of Charge (ASoC): The ability of a new battery to be charged in a given period.
State of Health (SoH)
It reflects battery performance by examining capacity, current output, voltage, and self-discharge, expressed as percentages.
Sulfation
The formation or deposition of lead sulfate on the surface and in the pores of the active material of a battery’s lead plates. If sulfation is excessive and large crystals form on the plates, the battery will not work efficiently.
Thermal runaway
A condition in which a battery or battery pack destroys itself due to internal heating while charging at a constant potential.
Valve Regulated Lead Acid (VRLA) Batteries
See “SLA Batteries
Wattage
A unit of power, amperage (A) times volts (V), equals watts (W).
Watt-Hour
A unit of electrical energy equals 1 watt of electricity consumed in 1 hour. Battery voltage (V) multiplied by rated capacity (Ah) yields battery energy (Wh).