Lithium batteries have become a crucial power source *for* modern electronics. Whether it’s powering your smartphone, laptop, electric vehicle, or power tools, lithium batteries are ubiquitous. But have you ever wondered what components are inside these batteries and how they work? This article will take you on a deep dive into lithium batteries, exploring their components and their functions, to give you a deeper understanding of the chemistry behind them.
Part 1: Types of Lithium Batteries
Lithium batteries come in various types, each with distinct *chemical compositions* and components. Each type has its advantages and disadvantages, making them suitable for specific applications. Here are six common types of lithium batteries:
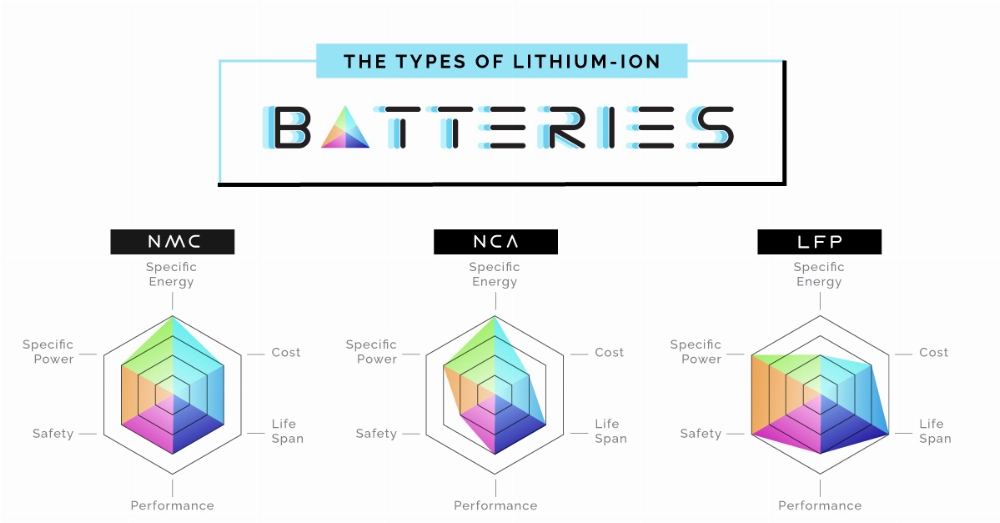
1. Lithium Iron Phosphate (LiFePO4)
Lithium Iron Phosphate batteries, also known as LFP, are known for their excellent thermal stability and long lifespan. LFP batteries use *lithium iron phosphate* as the cathode material, while graphite is used as the anode. LFP batteries typically have a nominal voltage of 3.2V, but can provide 12.8V if connected in series. These batteries are widely used in electric vehicles and energy storage systems due to their safety and longevity.
2. Lithium Cobalt Oxide (LiCoO2)
Lithium Cobalt Oxide batteries are known for their high energy density, compact size, and lightweight design. In these batteries, Lithium Cobalt Oxide (LiCoO2) acts as the cathode material, while graphite serves as the anode. While LCO batteries have a high energy density, they also raise some concerns regarding safety and lifespan. They typically offer 500 to 1000 charge cycles and are commonly used in smartphones, laptops, cameras, and tablets.
3. Lithium Manganese Oxide (LiMn2O4)
Lithium Manganese Oxide batteries use Lithium Manganese Oxide as the cathode and graphite as the anode. These battery *cells* form a 3D structure, which ensures smooth ion flow and minimal resistance. This design enhances thermal stability and safety. However, LMO batteries have a shorter lifespan, typically between 300 and 700 cycles, making them well-suited for use in medical devices, power tools, and some hybrid electric vehicles.
4. Lithium Nickel Manganese Cobalt Oxide (NMC)
Lithium Nickel Manganese Cobalt Oxide batteries combine the properties of nickel, manganese, and cobalt to provide a high-performance, stable battery. Nickel and cobalt offer high energy density, while manganese adds stability, making the battery reliable and suitable for electric vehicles, power tools, and portable devices.
5. Lithium Nickel Cobalt Aluminum Oxide (NCA)
Lithium Nickel Cobalt Aluminum Oxide batteries strike a balance between high energy density and power output. Due to the combination of nickel, cobalt, and aluminum, these batteries have a long lifespan. NCA batteries are commonly used in electric vehicles and are known for their ability to deliver consistent performance over time.
6. Lithium Titanate (LTO)
Lithium Titanate batteries are unique because they use lithium titanate instead of graphite as the anode. The *positive electrode* can be made from LMO or NMC materials. LTO batteries support rapid charging and perform well in extreme temperatures, making them suitable for electric vehicles, power plants, and uninterruptible power supplies (UPS). However, their energy density is generally lower, which results in increased battery weight.
Part 2: Components of Lithium Batteries
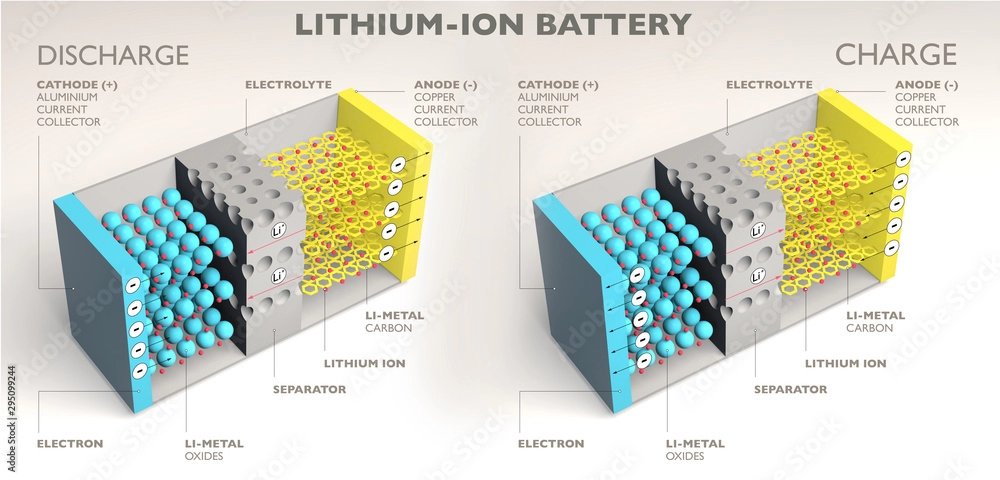
Lithium batteries consist of several electrochemical components that work together to store and deliver electrical energy. These components are the anode (-), cathode (+), electrolyte, and separator, all of which are encased in a battery *case* to ensure proper operation.
1. Cathode (+)
The cathode is the positive electrode in a lithium battery and is typically made of a lithium-containing metal oxide. The material used for the cathode can vary depending on the type of lithium battery, such as Lithium Cobalt Oxide (LiCoO2), Lithium Iron Phosphate (LiFePO4), or Nickel Manganese Cobalt *Oxide* (NMC). The cathode’s role is to facilitate the release and reception of lithium ions during charge and discharge cycles.
2. Anode (-)
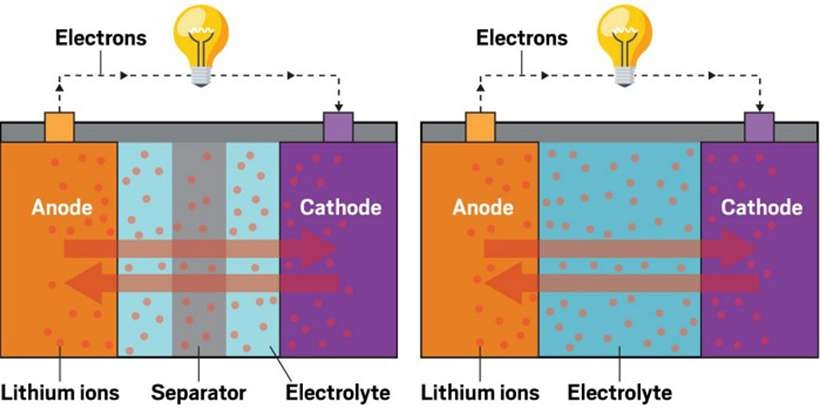
The anode is the negative electrode of the battery. It is typically made of graphite or silicon-based materials. During the discharge cycle, lithium ions move from the anode to the cathode, releasing energy. The anode also plays a crucial role in battery charging, when lithium ions move back from the cathode to the anode.
3. Electrolyte
The electrolyte is the medium through which lithium ions travel between the cathode and anode during charging and discharging. It is typically a lithium salt dissolved in a solvent, which ensures ion flow while preventing the movement of electrons within the battery.
4. Separator
The separator is a crucial component that prevents direct contact between the cathode and anode, which could otherwise lead to a short circuit. It is typically made of a porous, non-conductive material that allows ions to pass through while maintaining the battery’s safety.
With an understanding of the various types and components of lithium batteries, you can now have a clearer picture of how they function and how they power *so many* modern devices. Whether it’s electric vehicles, smartphones, or power tools, lithium batteries are a powerful and efficient source of energy that will continue to shape technological advancements.
For more detailed insights about reviving unchargeable lithium-ion batteries, check out our expert guide.
Lithium Battery Composition and Manufacturing Process
Part 1: Key Components of a Lithium Battery
- Positive Electrode (Cathode)
正极 (Positive Electrode/Cathode) is a crucial element in a lithium-ion battery. Serving as the positive terminal, it typically consists of a metal oxide, such as lithium cobalt oxide, and stores lithium ions during the charging process. During discharge, these lithium ions move from the positive electrode to the negative electrode and return to the positive electrode when recharged. - Negative Electrode (Anode)
负极 (Negative Electrode/Anode) is the negative terminal in a lithium-ion battery. It primarily uses graphite as its material. During discharge, lithium ions move from the positive electrode to the negative electrode and are embedded within the crystal structure of the negative electrode. This movement facilitates the release of electrical energy. - Electrolyte
The electrolyte in a lithium battery is a lithium salt dissolved in an organic solvent. Its main purpose is to enable the efficient movement of lithium ions between the positive and negative electrodes to reduce internal resistance and ensure the battery’s lasting performance. - Separator
The separator is a thin, microporous plastic film located between the positive and negative electrodes. Its role is to physically separate the positive and negative battery components, preventing any short circuits while allowing lithium ions to flow freely.
Part 2: Lithium Battery Manufacturing Process
The production of lithium batteries involves several crucial steps, each requiring meticulous attention to detail and adherence to strict protocols.
Step 1: Electrode Preparation
Electrodes, consisting of positive and negative electrode materials, are made using materials including lithium compounds, cobalt oxide, and graphite. Depending on the battery chemistry type, these electrodes are synthesized with conductive additives and binders to form a slurry. The slurry is then coated onto thin metallic foils, where aluminum is used on the positive electrode side and copper on the negative electrode side. After coating, the electrodes are dried to ensure proper consistency.
Step 2: Electrode Cutting and Stacking
At this stage, electrode sheets are cut into precise dimensions using specialized machinery. After cutting, the positive and negative electrodes are stacked together with the separator. This careful stacking prevents short circuits and ensures battery integrity.
Step 3: Electrolyte Filling
The stacked electrodes and separator are placed inside the battery casing. A liquid electrolyte, consisting of lithium salts dissolved in an organic solvent, is carefully injected into the assembly to saturate the electrodes and separator, thus enabling smooth ion transfer during charging and discharging.
Step 4: Sealing
Once the battery components are stacked and the electrolyte has been added, the final sealing process begins. The battery is encapsulated in a laminated film, and heat is applied around the edges to create a secure seal, preventing leakage.
Step 5: Formation, Cycling, and Testing
At this stage, the battery undergoes initial charging and discharging cycles, which are crucial for establishing a stable solid electrolyte interphase (SEI). This process activates the battery and verifies that all components are functioning correctly. Rigorous testing is conducted to check various parameters, such as power, capacity, safety, and cycle life.
Part 3: Assembling a Lithium Battery Pack
To create a fully functional battery pack, multiple lithium cells need to be assembled together. The process begins by arranging the individual cells in a rack, connecting each cell to its neighbors via positive and negative terminals. The required number of cells depends on the desired battery capacity. For example, to make a 50Ah battery pack, 15 individual cells might be needed.
After welding, a battery management system (BMS) is installed. The BMS ensures optimal operation of each cell and coordinates the overall function of the battery pack. Finally, the battery pack undergoes extensive testing, similar to that of individual cells, to verify performance and safety before market approval.
Part 4: Lithium Battery Safety Testing
All lithium batteries must undergo rigorous safety testing to ensure their reliability and safety. The Battery Management System (BMS) plays a crucial role in monitoring battery health and preventing unsafe operating conditions. Common safety tests for lithium batteries include:
- Overcharge Test: Ensures that the battery does not overcharge, which can lead to overheating or other issues.
- Over-discharge Test: Evaluates the battery’s performance when discharged beyond its rated capacity.
- Short Circuit Test: Ensures that the battery can withstand a short circuit without failing.
- Mechanical Shock Test: Tests the battery’s resilience under physical stress or impact.
- Thermal Runaway Test: Evaluates the battery’s behavior under high-temperature conditions.
- Thermal Abuse Test (Thermal Stress Test): Checks the battery’s performance under extreme temperature fluctuations.
- Impact and Vibration Test: Determines whether the battery can withstand rough handling during transport or use.
These tests ensure that the battery performs optimally and remains safe under various challenging conditions.
Frequently Asked Questions (FAQs)
What are the main components of a lithium battery pack?
Lithium battery packs mainly consist of individual lithium-ion or lithium-polymer cells, a Battery Management System (BMS), cell interconnects, a thermal management system, and an enclosure to house the battery pack.
What is the difference between lithium-ion and lithium-polymer batteries?
The main difference lies in the electrolyte. Lithium-ion batteries use a liquid electrolyte, while lithium-polymer batteries use a gel polymer electrolyte. In terms of safety, lithium-polymer batteries are generally considered more stable and safer than lithium-ion batteries.
Can lithium batteries overcharge or overheat?
Yes, lithium batteries are susceptible to overcharging and overheating, especially if the Battery Management System (BMS) malfunctions or an unsuitable charger is used. Overcharging and overheating can damage the battery and even lead to explosions.
What is the role of the separator in a lithium-ion cell?
The separator is a thin, porous membrane that acts as a safety barrier between the positive and negative electrodes. It is a critical component for preventing short circuits, thereby ensuring the safety and correct operation of the battery.
How does a BMS monitor and control a lithium battery pack?
A Battery Management System (BMS) is essential for maintaining the battery pack. It ensures the optimal performance of all components by balancing cells, managing charging, monitoring temperature, and preventing overcharging and over-discharging.
Okay, I need the original content to proceed with the rewriting. Can you provide the text you would like me to rewrite? For example, if you would like to learn more about different facets of lithium-ion batteries, you can check out our comprehensive guide on Lithium Iron Phosphate (LiFePO4) batteries for a deeper understanding of their chemistry and applications.