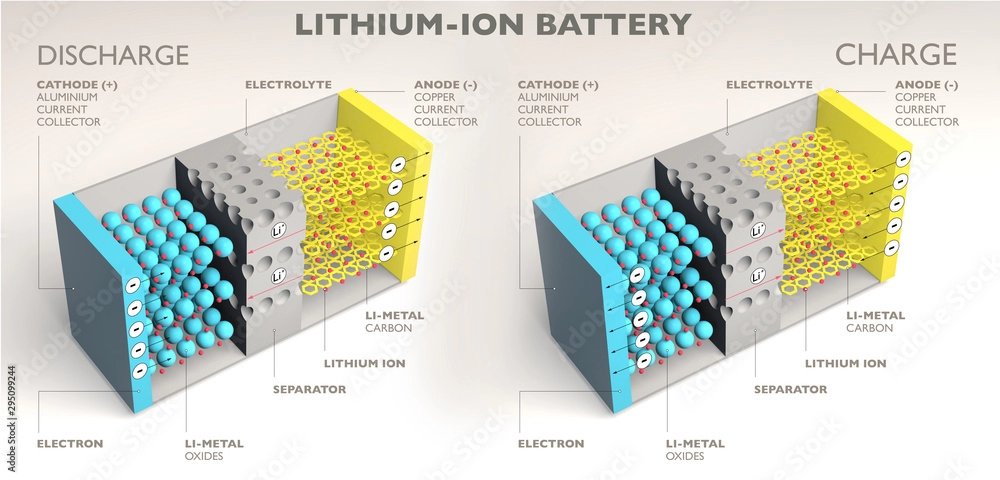
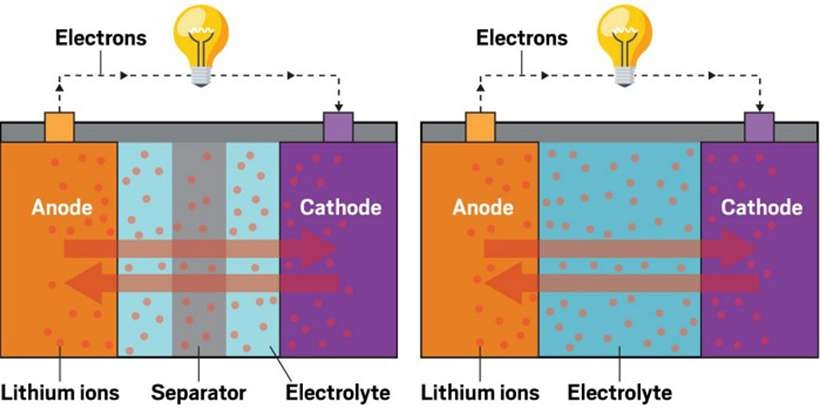
Batteries are an indispensable part of our daily lives, powering everything from smartphones and laptops to electric vehicles and renewable energy systems. In this article, we will explore the key components of a battery and discuss the elements found in different types of batteries.
Part 1: What’s Inside a Battery?

Anode Materials
Cathode materials often involve compounds such as metal oxides or phosphates to enhance conductivity, stability, and compatibility. These properties can be enhanced through doping or modification.
Lithium Cobalt Oxide
Lithium cobalt oxide was the first cathode material used in commercial lithium batteries. It has a high capacity and voltage but faces challenges such as high cost and safety concerns. Over time, other materials have begun to replace it.
Lithium Manganese Oxide
Lithium manganese oxide is a low-cost and safer cathode material, making it ideal for low-power and energy storage batteries. However, it suffers from capacity decay and limited temperature range, requiring doping or modification to improve performance.
Lithium Iron Phosphate
Lithium iron phosphate is known for its safety and charge-discharge cycle life, making it suitable for high-power and energy storage batteries. While it has excellent safety characteristics, it has lower capacity and poor conductivity, requiring doping or modification to enhance performance. Learn more about why lithium iron phosphate batteries get more market share.
Ternary Materials
Ternary materials such as Nickel Cobalt Aluminum (NCA) and Lithium Nickel Cobalt Manganese (NCM) have high capacity and energy density. They are mainly used in high-power batteries but face challenges such as high cost, safety concerns, and short cycle life, requiring doping or compounding to improve their performance.
Anode
Anode materials use metallic or non-metallic compounds to store lithium through reversible intercalation or conversion reactions with lithium ions.
Carbon-Based
Carbon-based anodes are commonly used in lithium batteries due to their low cost, low voltage, and stable cycling performance. They are commonly found in small electronics and power batteries. Carbon-based materials such as natural and artificial graphite form a LiC6 structure to store lithium, with a theoretical capacity of 372 mAh g⁻¹.
Silicon-Based
Silicon-based anodes have high capacity (4200 mAh g⁻¹) and high energy density, making them ideal for high-performance power and energy storage batteries. This material, including crystalline silicon and silicon oxide, stores lithium through reversible alloying or conversion reactions.
Lithium Titanate Anode (LTO)
Lithium titanate is a safe and durable anode material used in high-power batteries. Its spinel structure allows for the reversible insertion and extraction of Li⁺ ions, forming a Li₄Ti₅O₁₂ structure with a theoretical capacity of 175 mAh g⁻¹.
Lithium Metal Anode
Lithium metal is considered an ideal anode material because it has the highest theoretical capacity (3860 mAh g⁻¹), the lowest operating voltage, and high energy density. It is mainly used in advanced lithium-sulfur and lithium-air batteries, storing lithium through reversible deposition and dissolution with Li⁺ ions.
Separator
The separator (membrane) is a critical component in lithium batteries that prevents the electrodes from contacting each other and causing a short circuit. It allows lithium ions to pass through during charging and discharging.
Common Separator Materials
Polypropylene (PP) and polyethylene (PE) are widely used as separator materials due to their low cost, high porosity, and stability, effectively preventing short circuits.
Organic Electrolyte
The electrolyte consists of lithium salts and organic solvents, which facilitates ion transport in the battery. It plays a crucial role in enabling the charging and discharging processes of lithium batteries.
Part 2: What Elements are in Different Batteries?
Lithium-Ion Batteries
Lithium-ion batteries consist of several key components, with lithium being the primary active material in both the cathode and anode. During discharge, lithium ions move from the anode to the cathode through the electrolyte, generating electrical energy.
Elements: Cathode
Lithium-Ion Batteries
Lithium-ion (Li-ion) batteries are widely used in various devices and applications. Key materials in these batteries include lithium cobalt oxide, lithium iron phosphate, and other lithium-based compounds for the positive electrode. The negative electrode is typically made of graphite.
Applications: Lithium-ion batteries are known for their impressive energy density, long cycle life, and ability to power a wide range of devices, from smartphones to electric vehicles.
Nickel-Cadmium (NiCd) Batteries
Nickel-cadmium (NiCd) batteries are reliable and versatile, widely used in various devices. During discharge, cadmium at the negative electrode undergoes oxidation, releasing electrons and allowing current to flow. Nickel oxide in the positive electrode is reduced as it accepts these electrons.
Elements: The positive electrode is made of nickel hydroxide, and the negative electrode is cadmium. The electrolyte is typically an alkaline solution containing potassium hydroxide.
Applications: Nickel-cadmium batteries are known for their reliability and durability, making them suitable for cordless phones, portable power tools, and more, etc.
Other Battery Types
While lithium-ion and nickel-cadmium batteries are well-known, many other battery types use different materials for different applications. Notable examples include:
1. Lead-Acid Batteries
Elements: Lead-acid batteries use a sulfuric acid solution as the electrolyte and feature lead dioxide (positive electrode) and lead sponge (negative electrode).
Applications: These batteries are commonly used in vehicles, uninterruptible power supplies (UPS), and backup power systems.
2. Zinc-Carbon Batteries
Elements: Zinc-carbon batteries feature a zinc negative electrode and a manganese dioxide positive electrode, using an ammonium chloride solution as the electrolyte.
Applications: These batteries are common in household devices such as remote controls and flashlights.
3. Nickel-Metal Hydride (NiMH) Batteries
Elements: Nickel-metal hydride batteries consist of a nickel(III) hydroxide positive electrode and a hydrogen-absorbing alloy negative electrode.
Applications: NiMH batteries, known for their rechargeability, are often used in toys, digital cameras, and hybrid vehicles.
4. Alkaline Batteries
Elements: Alkaline batteries feature a manganese dioxide positive electrode and a zinc negative electrode, along with an alkaline electrolyte such as potassium hydroxide.
Applications: These batteries are widely used in various consumer electronics.
Conclusion
Batteries play a crucial role in modern life by powering numerous devices and applications. Key elements used in batteries, including lithium, lead, nickel, and other materials, are essential for providing energy and ensuring the functionality of our devices.