The terms battery cell and battery are often used interchangeably, but they refer to different components within an energy storage system. Understanding the differences between them is crucial, especially when choosing the right power source. This article will explore the composition, types, and key distinctions between battery cells and batteries.
Part 1: What is a Battery Cell?
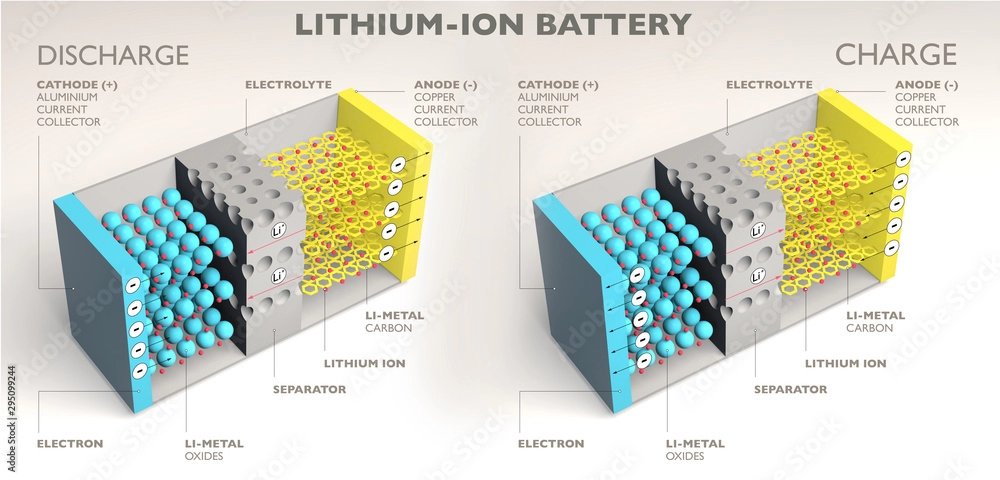
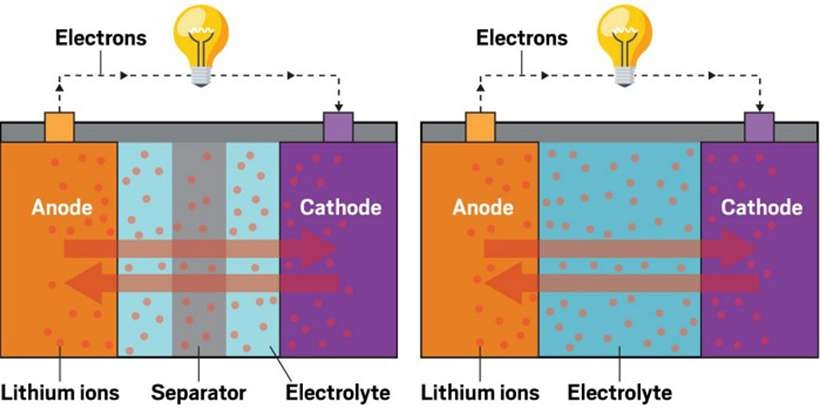
In the context of energy storage, a battery cell is the basic unit that converts chemical energy into electrical energy. Battery cells are the building blocks of batteries, and they can function independently or be combined to form larger energy storage systems.
Composition of a Battery Cell
A typical battery cell consists of several essential components, including:
- Anode: The anode is the electrode from which current (electrons) flows out of the cell during discharge. It is typically made of an active material that undergoes oxidation.
- Cathode: The cathode is the electrode where reduction occurs during discharge. It is usually composed of a material that readily accepts electrons during discharge.
- Electrolyte: The electrolyte is a medium that facilitates the transport of ions between the anode and cathode, enabling the chemical reactions necessary for chemical energy production.
- Separator: The separator acts as a physical barrier between the anode and cathode, preventing direct contact and short circuits while still allowing ions to pass through.
Types of Battery Cells
Battery cells can be classified based on their chemistry and application. Some common types include:
- Primary Cells: These are non-rechargeable cells that provide electrical energy once until their chemical reactants are depleted. Primary cells are commonly used in disposable batteries for devices like remote controls and flashlights.
- Secondary Cells: Secondary cells are rechargeable and can be returned to their original state by applying an external electric current. They are commonly used in devices like smartphones and electric vehicles due to their reusability.
- Alkaline Cells: Alkaline cells use an alkaline electrolyte, typically potassium hydroxide (KOH). They are known for their relatively long shelf life and stable voltage output, making them ideal for everyday devices.
- Lithium-Ion Cells: Lithium-ion cells have gained popularity for their high energy density, lightweight design, and long charge retention. These cells are widely used in portable electronics, electric vehicles, and renewable energy systems. For more details, check out this Lithium-Ion Battery Guide.
Part 2: What is a Battery?
While a battery cell is a single energy storage unit, a battery consists of multiple cells connected in series or parallel to provide a higher voltage or current output. A battery is essentially an assembly of cells that stores electrical energy.
Composition of a Battery
A battery is made up of multiple cells connected in series or parallel, depending on the desired voltage and capacity. The key components of a battery are:
- Cells: As mentioned earlier, a battery consists of one or more cells connected together.
- Terminals: Terminals serve as the connection points between the battery and the electrical device it powers. They allow the flow of electric current into and out of the battery.
- Casing: The casing provides physical protection and structural support for the cells and other internal components of the battery.
- Battery Management System: Advanced batteries may include a management system that monitors and controls factors such as temperature, voltage, and charging/discharging to optimize performance.
Types of Batteries
Batteries come in various forms to meet the needs of different applications. Some popular types include:
- Lithium-Ion Batteries: Known for their high energy density and rechargeability, lithium-ion (Li-ion) batteries have revolutionized the portable electronics and electric vehicle industries. They are the preferred choice for devices like smartphones, laptops, and electric cars.
- Lead-Acid Batteries: Lead-acid batteries are one of the oldest and most widely used types. They are commonly used in applications such as automobiles and uninterruptible power supplies (UPS).
Common Battery Technologies
Battery technology plays a crucial role in various applications, from automobiles to renewable energy systems. Here are some of the most widely used battery technologies:
Lead-Acid Batteries
Lead-acid batteries are one of the oldest and most widely used types. Their reliable performance and relatively low cost make them commonly used in automotive applications, uninterruptible power supplies (UPS), and renewable energy systems.
Nickel-Cadmium Batteries
Nickel-cadmium (NiCd) batteries are known for their good cycle life and high discharge rate. They are often used in portable power tools, emergency lighting systems, and various other industrial applications where a stable power supply is required.
Nickel-Metal Hydride Batteries
Nickel-metal hydride (NiMH) batteries have gained popularity in consumer electronics due to their higher energy density compared to NiCd batteries. They are widely used in devices such as digital cameras, laptops, and cordless phones, offering longer runtimes and improved performance.
Part 3: Cell vs. Battery: Understanding the Difference
When dealing with energy storage systems, it’s crucial to understand the key differences between cells and batteries. Here’s a breakdown:
Composition
A cell consists of two electrodes and an electrolyte. On the other hand, a battery is made up of multiple interconnected cells.
Function
A cell can operate independently, while a battery requires multiple cells working together to store and produce electricity.
Voltage and Capacity
Typically, cells have lower voltage and capacity compared to batteries. This is because batteries, consisting of multiple cells, provide a combined higher voltage and current.
Applications
Cells are often used in small, low-power devices, while batteries have a wide range of applications, including automotive, electronics, and renewable energy.
Shape
Cells are often smaller and more compact, while batteries come in various sizes and shapes, from cylindrical cells to flat pouch designs.
Energy Density
Batteries generally have a higher energy density than individual cells, allowing them to store more energy for longer use.
Rechargeability
Many cells are rechargeable, but batteries are almost always designed to be rechargeable, allowing for repeated charging.
Complexity
Batteries are more complex than individual cells due to the interconnection of multiple cells. They require additional circuitry and management systems for proper operation.
Part 4. Frequently Asked Questions
How many cells are in a battery?
The number of cells in a battery can vary depending on its design and intended use. Some batteries consist of a single cell, while others may contain multiple cells connected in series or parallel to enhance its voltage or capacity.
What is the major difference between cells and batteries?
A cell is the basic unit that generates electrical energy, while a battery is a collection of cells. The symbol for a cell is typically represented by two parallel lines, representing the electrodes. The longer line indicates the positive terminal, and the shorter line indicates the negative terminal. In contrast, the symbol for a battery includes multiple cells, often represented by a series of alternating long and short parallel lines, each pair representing a cell’s positive and negative terminals.
Is a lithium battery a cell?
No, a lithium battery is not a single cell. It refers to a type of battery that uses lithium-based chemistry within its cells. A lithium battery can contain one or more lithium cells, depending on the design and application.
Do two cells mean two batteries?
Not necessarily so. If the two cells are internally connected, they constitute one battery. However, if each cell is independent, then two cells mean two separate batteries. The distinction lies in the arrangement and connection of the cells within the battery design.
Battery Arrays vs. Single Batteries: Which is Better for Your Energy Needs?
Understanding the differences between battery arrays and single batteries helps you choose the ideal solution for your energy needs. This article will introduce the workings of each, their respective advantages, and how to achieve the best balance of performance and cost for your needs.
What is a Battery Array?
A battery array consists of multiple interconnected batteries designed to provide stable and reliable power. This article will explore different types of battery arrays, analyze their advantages, and why they are ideal for meeting various energy needs.
Growth Trends in the Flexible Thin-Film and Printed Battery Market
The flexible thin-film and printed battery market is experiencing rapid growth. This article will introduce the industry’s latest developments, innovations, and challenges, and look forward to future trends shaping the market.
Battery Charging Cycles: Extending Battery Life and Optimizing Battery Performance
Battery charging cycles are crucial for a battery’s lifespan and efficiency. This article will explain how to extend battery life and optimize battery performance, including using tools like battery charging calculators.
Understanding 11.1V LiPo Batteries: Characteristics, Advantages, and Common Applications
11.1V LiPo batteries are commonly used to power remote control models, drones, and other electronic devices. This guide will explore the battery’s characteristics, advantages, and typical applications.