As technology advances rapidly, various battery types have emerged, each catering to specific needs. Among the most commonly used are Ni-MH (Nickel-Metal Hydride), Li-Ion (Lithium-Ion), and Ni-Cd (Nickel-Cadmium) batteries. In this article, we will delve into their unique characteristics, including their chemical composition, structure, advantages, disadvantages, applications, and provide a comparative analysis of Ni-MH and Li-Ion batteries compared to Ni-Cd batteries.
Part 1: Ni-MH Batteries
Chemical Composition and Structure
Ni-MH batteries use nickel oxide-hydroxide (NiOOH) as the positive electrode and a hydrogen-absorbing alloy as the negative electrode. The electrolyte consists of an alkaline solution, typically potassium hydroxide (KOH). Ni-MH batteries are constructed by stacking multiple cells, with each cell containing a positive electrode plate, a negative electrode plate, and a separator in between.
Advantages
- Higher Energy Density: Ni-MH batteries provide longer run times than Ni-Cd batteries.
- No Memory Effect: Partial discharge does not reduce the overall battery capacity.
- Environmentally Friendly: Unlike Ni-Cd batteries, Ni-MH batteries do not contain toxic cadmium.
- Lower Self-Discharge Rate: Ni-MH batteries can hold their charge for longer periods when not in use.
Disadvantages
- Lower Energy Density: Compared to Li-Ion batteries, Ni-MH batteries store less energy, resulting in shorter run times.
- Higher Self-Discharge Rate: While still lower than Ni-Cd batteries, it is higher than Li-Ion batteries.
- Voltage Depression: They are prone to voltage drops if not properly maintained.
- Relatively Higher Cost: Ni-MH batteries tend to be more expensive than Ni-Cd batteries.
Applications
Ni-MH batteries are widely used in various applications, such as:
- Portable Electronics: Digital cameras, handheld gaming consoles, and MP3 players.
- Hybrid and electric vehicles.
- Cordless Power Tools.
- Emergency Backup Systems.
- Renewable Energy Storage.
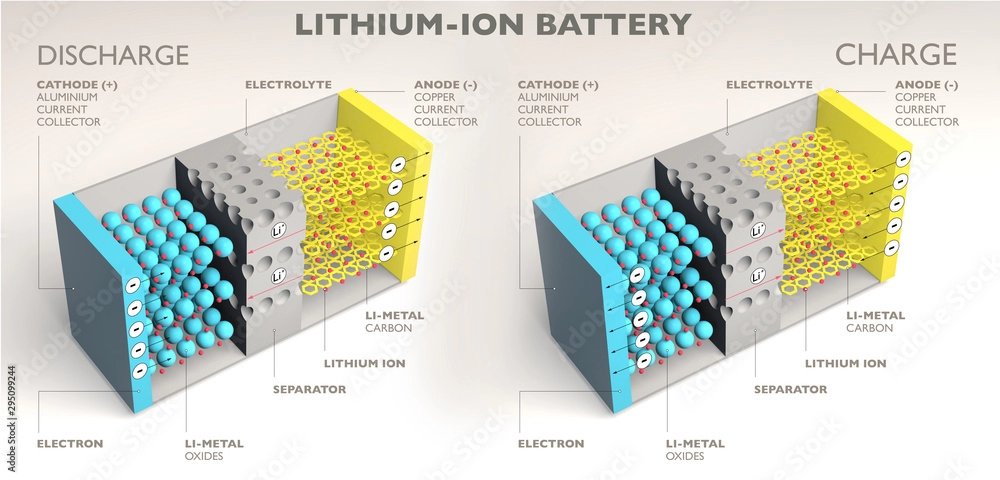
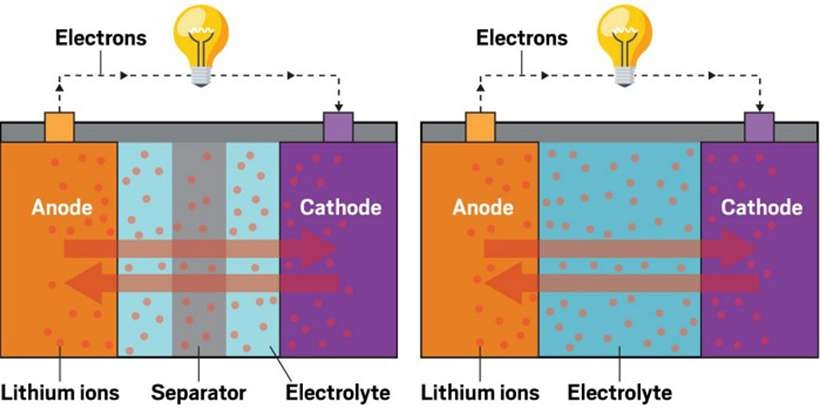
Part 2: Li-Ion Batteries
Chemical Composition and Structure
Li-Ion batteries utilize lithium compounds, typically lithium cobalt oxide (LiCoO2), as the positive electrode and carbon-based materials as the negative electrode. The electrolyte is a lithium salt dissolved in an organic solvent. Similar to Ni-MH batteries, multiple battery cells are stacked together in Li-Ion batteries.
Advantages
- High Energy Density: Li-Ion batteries offer significantly longer run times compared to Ni-MH and Ni-Cd batteries.
- No Memory Effect: Partial discharge does not degrade battery performance.
- Low Self-Discharge Rate: They retain their charge better than Ni-MH batteries.
- Lightweight and Compact: Li-Ion batteries are small and lightweight, making them ideal for portable devices.
Disadvantages
- Higher Cost: Li-Ion batteries are generally more expensive than Ni-MH and Ni-Cd batteries.
- Temperature Sensitivity: High temperatures can negatively affect performance and lifespan.
- Requires Protection Circuitry: Li-Ion batteries require built-in protection circuits to prevent overcharging and over-discharging.
- Aging and Capacity Loss: Li-Ion batteries degrade over time, requiring eventual replacement.
Applications
Li-Ion batteries play an integral role in numerous devices and industries, such as:
- Smartphones, Tablets, and Laptops.
- Electric Vehicles.
- Uninterruptible Power Supplies (UPS).
- Aerospace.
- Renewable Energy Storage Systems.
Part 3: Ni-Cd Batteries
Chemical Composition and Structure
Ni-Cd batteries employ nickel hydroxide (Ni(OH)2) as the positive electrode and cadmium (Cd) as the negative electrode. The electrolyte is a potassium hydroxide (KOH) solution. These batteries are constructed by connecting individual cells in series or parallel to achieve the desired voltage and capacity.
Advantages
- High Discharge Rate: Ni-Cd batteries are suitable for applications that require high power bursts.
- Temperature Resistance: They are resistant to extreme temperatures.
- Long Lifespan: Ni-Cd batteries can withstand a large number of charge and discharge cycles.
- Lower Cost: They are more affordable compared to Ni-MH and Li-Ion batteries.
Disadvantages
- Memory Effect: Incomplete discharge before recharging reduces their overall capacity.
- Toxic Cadmium: The presence of cadmium raises environmental concerns, especially during disposal or recycling.
- Higher Self-Discharge Rate: Ni-Cd batteries tend to lose their charge more quickly compared to Ni-MH and Li-Ion batteries.
Conclusion: Comparative Analysis
In comparing Ni-MH and Li-Ion batteries against Ni-Cd batteries, it is evident that each type has its advantages and disadvantages. While Ni-MH and Li-Ion batteries do not suffer from the memory effect and are more environmentally friendly than Ni-Cd batteries, they present trade-offs in energy density, cost, and lifespan. Ni-Cd batteries, although more affordable and capable of high discharge rates, are plagued by the memory effect and environmental concerns due to the presence of cadmium. Ultimately, the choice between these batteries depends on the specific application requirements, such as cost, energy needs, and environmental impact.
Therefore, a thorough understanding of these battery characteristics helps inform decisions when selecting batteries for various devices or industries.
Nickel-Cadmium (NiCad) Battery Overview
Nickel-cadmium (NiCad) batteries are a type of rechargeable battery made using nickel hydroxide and metallic cadmium. These batteries are known for their reliability and durability, but they also have certain limitations.
Advantages of NiCad Batteries:
- Durability: NiCad batteries are rugged and can withstand a significant number of charge and discharge cycles.
- High Discharge Rate: These batteries can efficiently deliver high discharge currents, making them suitable for devices that require a large amount of power in a short time.
- Temperature Resistance: NiCad batteries perform well in extreme temperature conditions, both hot and cold.
Disadvantages of NiCad Batteries:
- Memory Effect: A major drawback is their memory effect, where partial discharges can cause the battery to “remember” the lower capacity, reducing its overall performance. It is best to fully discharge them before each charge to maintain optimal capacity.
- Environmental Impact: Cadmium is a toxic substance, making NiCad batteries environmentally hazardous and difficult to dispose of properly.
- Lower Energy Density: Compared to other battery technologies like Nickel-Metal Hydride (NiMH) and Lithium-Ion (Li-Ion), NiCad batteries have a lower energy density, resulting in shorter battery runtime.
Common Applications of NiCad Batteries
NiCad batteries are widely used in various devices and industries, including:
- Cordless power tools
- Two-way radios
- Emergency lighting systems
- Medical equipment
- Hobbyist and consumer electronics
Comparison: NiCad vs. NiMH and Li-Ion Batteries
When comparing NiCad batteries to NiMH and Li-Ion batteries, several key factors must be considered. Here’s a detailed comparison of these battery types:
Energy Density
- Li-Ion Batteries: These batteries offer the highest energy density, providing longer runtime compared to NiMH and NiCad batteries.
- NiMH Batteries: While slightly lower than Li-Ion, NiMH batteries still offer a respectable energy density.
- NiCad Batteries: These batteries have the lowest energy density, resulting in shorter runtime compared to NiMH and Li-Ion batteries.
Memory Effect
- Li-Ion and NiMH Batteries: These battery types do not exhibit a memory effect, allowing for partial discharges without negatively impacting performance.
- NiCad Batteries: NiCad batteries, on the other hand, suffer from the memory effect, requiring full discharges before charging to maintain their full capacity.
Self-Discharge Rate
- Li-Ion Batteries: Li-Ion batteries have the lowest self-discharge rate, meaning they hold their charge longer when not in use.
- NiMH Batteries: NiMH batteries have a lower self-discharge rate compared to NiCad batteries, but they still lose charge faster than Li-Ion batteries.
- NiCad Batteries: These batteries have the highest self-discharge rate, meaning they lose charge faster when not in use.
Environmental Impact
- NiMH Batteries: These batteries are more environmentally friendly than NiCad batteries as they don’t contain toxic cadmium.
- Li-Ion Batteries: While also cadmium-free, the environmental impact of Li-Ion batteries mainly lies in the extraction of lithium and disposal, requiring proper recycling measures.
Cost
- NiCad Batteries: Generally more budget-friendly.
- NiMH Batteries: Slightly more expensive than NiCad batteries but cheaper than Li-Ion batteries.
- Li-Ion Batteries: The most expensive but offer higher performance benefits.
Applications
- NiMH Batteries: Are used in portable electronics, hybrid and electric vehicles, cordless power tools, emergency backup systems, and renewable energy storage.
- Li-Ion Batteries: Found in smartphones, laptops, electric vehicles, UPS systems, aerospace applications, and renewable energy storage.
- NiCad Batteries: Commonly used in cordless power tools, two-way radios, emergency lighting systems, medical equipment, and consumer electronics.